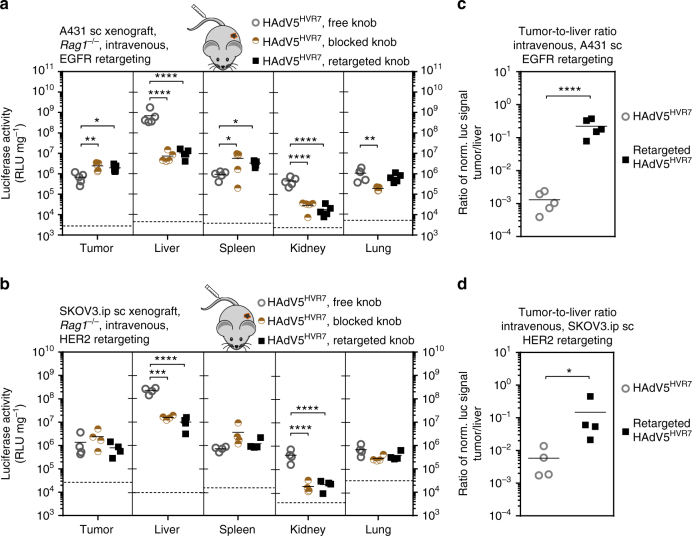
Fig. 4.
Adapter reduces off-targeting and increases tumor–liver ratio after intravenous (IV) injection. a, b 3 × 106 HAdV5HVR7 particles were injected in the tail vein of Rag1-/-mice, bearing either EGFR- or HER2-overexpressing subcutaneous tumors. Gene delivery was analyzed 48 hpi by luciferase activity, normalized to total protein amount. a More than 99% of the transgene activity was located in the liver after IV application of the virus alone. The blocking of the fiber knob by either adapter significantly decreased the gene delivery to liver, kidney and lung. Either knob-binding adapter increased the gene delivery to the tumor and the spleen. Background signals from control injections with PBS are indicated by dashed lines (each symbol represents one organ, n=5, two-way ANOVA of log-transformed data, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). b Intravenous injection of blocked, non-targeted HAdV5HVR7, and HER2-targeted HAdV5HVR7 resulted in strong reduction of liver and kidney transduction. A HER2 adapter-mediated targeting of the tumor was absent, since the luciferase activity was not higher than for the blocked virus. Background signals from control injections with PBS are indicated by dashed lines (each symbol represents one organ, n = 4, two-way ANOVA of log-transformed data, ***P < 0.001, ***P < 0.0001). c The ratio of transgene activity between tumor and liver was increased by the EGFR-retargeting adapter by a factor of 170 (each symbol represents the ratio of an individual mouse, two-sided, unpaired Welch’s t-test of log-transformed data, ****P < 0.0001). d The HER2-specific adapter increased the tumor–liver ratio in the SKOV3.ip xenograft by a factor of 25 (each symbol represents the ratio of an individual mouse, two-sided, unpaired Welch’s t-test of log-transformed data, *P < 0.05)