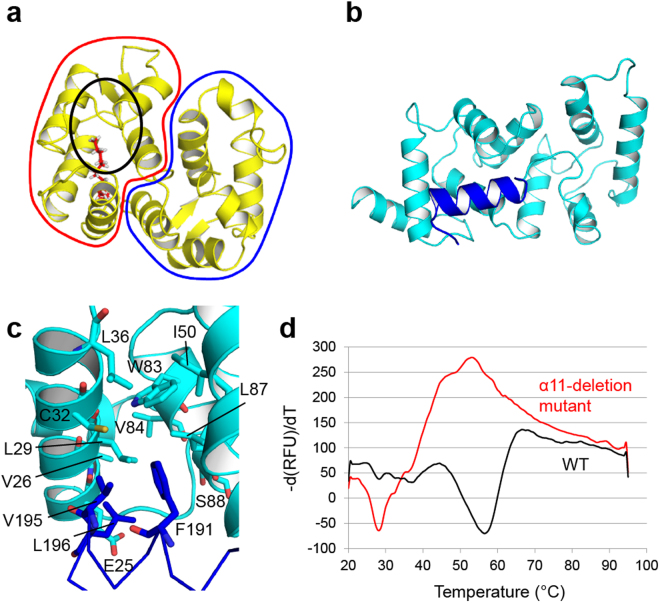
Figure 4.
Contribution of the α11 helix to the thermostability of calaxin. (a) Solution structure of recoverin in the Ca2+-free state (PDB ID: 1IKU). The N-terminal domain and C-terminal domain are surrounded by a red line and a blue line, respectively. The N-terminal myristoyl group is depicted in red sticks. The black circle shows the hydrophobic pocket interacting with the myristoyl group. (b) Crystal structure of Ca2+-bound calaxin in the open state. The α11 helix is colored in blue. (c) Hydrophobic interaction between the C-terminal α11 helix and N-terminal domain. The α11 helix is depicted in blue lines, and its hydrophobic residues (F191, V195 and L196) are shown as blue sticks. Residues forming the hydrophobic core in the N-terminal domain are depicted as cyan sticks. (d) Fluorescence-based thermal stability assay.