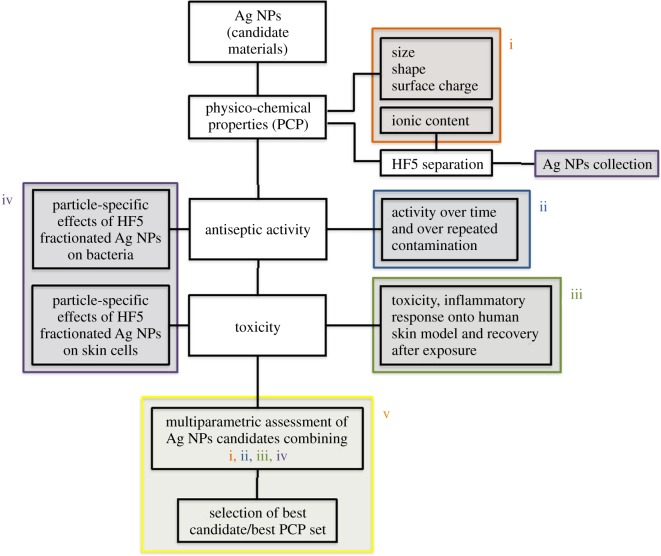
Figure 8.
Schematic representation of the multi-step approach used. (i) Characterization of the particles in suspension to match in vitro tests, (ii) testing of the nanoparticles to quantify their antibacterial response (acute and in a life-cycle scenario), (iii) in vitro test to assess toxic response upon contact (skin model), (iv) testing of collected, purified nanoparticle to assess particle-specific activity, and (v) correlation of relevant properties and nanoparticles activity (antiseptic/toxic and particle-specific).