Fig. 4.
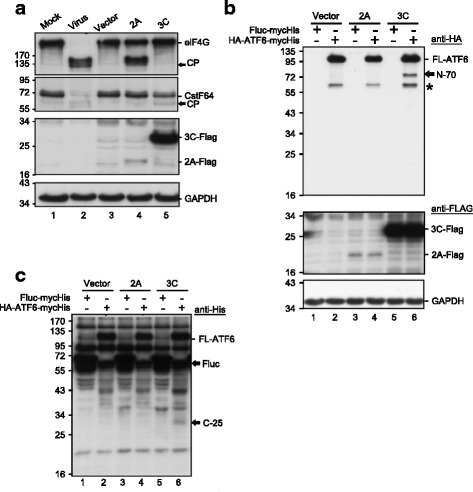
ATF6 was cleaved by viral-encoded proteases in HEK293T cells. The expression of recombinant 2Apro-FLAG and 3Cpro-FLAG in the AS3w vector was detected with anti-FLAG antibodies, and GAPDH was used as a loading control. a Validation of the protease catalytic activity of recombinant viral 2Apro and 3Cpro proteins. HEK293T cells were either infected with EV-A71 at an MOI of 200 as a positive control or were transfected with plasmids encoding 2Apro-FLAG or 3Cpro-FLAG for 24 h. The cleavage of endogenous eIF4G and CstF-64, substrates of viral 2Apro and 3Cpro, respectively, was assessed by immunoblotting using antibodies specific for eIF4G and CstF-64. The cleavage products (CP) of eIF4G and CstF-64 are indicated by arrows. b, c ATF6 was cleaved by viral 3Cpro but not by 2Apro. HEK293T cells were first transfected with a plasmid encoding HA-ATF6-mycHis or a control plasmid encoding FLuc-mycHis (pcDNA3.1/mycHis B(+)::FLuc). After 24 h, the cells were transfected with a vector control or plasmid encoding 2A-FLAG or 3C-FLAG. The cells were harvested and lysates were prepared 24 h post transfection. Equal amounts of cell lysates (30 μg/lane) were analyzed with 10% SDS-PAGE, followed by immunoblotting using antibodies against HA (b) or His tag (c) to detect the N- or C-terminal region of recombinant ATF6, respectively. The asterisk denotes a nonspecific autoproteolytic product. The data shown here are representative of at least three independent experiments
