Abstract
Background
In HIV patients using HAART insulin resistance is a central pathophysiological condition that can contribute to the development of diabetes and cardiovascular complications. To examine the role of adipocyte hormones and LPS in insulin resistance in HIV patients, we investigated the role of adiponectin, leptin, visfatin and LPS levels in the insulin resistance of HIV-infected patients treated with HAART.
Methods
This study included 67 HIV positive individuals on HAART and ten healthy controls. All participants performed plasma or serum levels of glucose; insulin; lipids, visfatin, leptin, adiponectin, and LPS. The homeostasis model assessment (HOMA-IR), was used to estimate insulin resistance.
Results
The levels of visfatin, leptin and adiponectin were similar between controls and HIV patients. However, circulating levels of LPS were higher in HIV patients on HAART than in controls. There was a positive correlation between LPS and TG (r = 0.49, p = 0.0001), between LPS and TG/HDL (r = 0.50, p = 0.0001), between LPS and insulin (r = 0.52, p = 0.0003), and between LPS and HOMA-IR (r = 0.52, p = 0.0005), in HIV patients.
Conclusions
Our results showed a clear correlation between plasma LPS and markers of insulin resistance, suggesting a relationship between LPS levels and metabolic alterations, particularly affecting lipids and insulin resistance in HIV patients.
Keywords: HIV, HAART, Insulin resistance, LPS
Background
Over the past 20 years, the introduction of antiretroviral therapy has increased the life expectancy of HIV patients, but it also introduced complicating diseases such as cardiovascular events, renal disease, osteoporosis and metabolic alterations [1–3]. Previous data showed that in patients with HIV in use of HAART the most prevalent metabolic alterations were reduced HDL-cholesterol and hypertriglyceridemia [4]. Among the metabolic complications encountered, insulin resistance is a central pathophysiological condition that can contribute to the development of diabetes and cardiovascular events [5–7]. In patients using HAART, the prevalence of insulin resistance ranges from 35 to 65%, which is higher than in a control population [8–10]. However, the mechanisms by which patients using HAART develop insulin resistance are not completely known [11, 12].
It is well known that changes in body fat distribution are a common finding in HIV-infected patients treated with HAART, associated with increased triglycerides and insulin resistance (IR) [13–21]. In the past, adipose tissue was considered only an energy storage organ, but over the last two decades, it has emerged as an endocrine organ secreting multiple hormones, proteins, and metabolites including inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and IL6 [22–26]. Some products produced by adipocytes (e.g., TNF-α, IL6) can induce insulin resistance. Few, as adiponectin can improve insulin action. Leptin shows a clear correlation with adipose mass. Visfatin plays a controversial role in the action of insulin on adipose tissue [27–29]. However, the role of these hormones in insulin resistance in HIV patients is not yet completely known.
In obesity/type 2 diabetes and other forms of insulin resistance, a subclinical inflammation can have a central role in the induction of insulin resistance [23–26]. This state of subclinical inflammation may be a consequence of alterations in the intestinal microbiota [21, 25–27] which may interfere with intestinal permeability, increasing the absorption of lipopolysaccharide (LPS) leading to increased activation of inflammatory pathways [26, 30–33]. Similarly, previous data showed that LPS circulating levels are increased in HIV patients and correlates with measures of innate immune activation [34, 35]. However, the role of LPS in insulin resistance in HIV patients is not completely known. To examine the role of adipocyte hormones and LPS in reduced insulin sensitivity in HIV patients, in the present study, we investigated the role of adiponectin, leptin, visfatin and LPS plasma levels in the insulin resistance of HIV-infected patients treated with HAART.
Methods
This was a cross-sectional study developed at the University State of Campinas, from January to July 2007.
Subjects
A total of 67 HIV positive individuals (47 men and 20 women; age 40.2 ± 9 years old) on HAART and 10 healthy controls adult (6 men and 4 women; age 34 ± 12.1 years old) were recruited at the Department of Internal Medicine, HIV/AIDS Clinical Research Unit of the Infectious Disease.
Inclusion criteria were age greater than 18 years, the presence of HIV infection (according to CDC 1993). All the HIV patients were on HAART. The HIV group was divided into two subgroups based on presence or absence of lipodystrophy, confirmed by a doctor or dietitian in the moment of data collected.
Exclusion criteria were subjects younger than 18 years old or older than 65, body mass index (BMI) > 27, steroids treatment, previous liposuction and similar cosmetic surgery, pregnancy, breastfeeding and diabetes type 1 and 2.
Anthropometric analysis
All participants underwent an anthropometric evaluation that included the nutritional status, BMI-calculated as weight/height2 (kg m2), skin fold, abdominal and hip circumferences. Waist circumference was measured to the nearest millimeter using anatomical landmarks.
The presence of lipodystrophy was evaluated according to the Multicenter Aids Cohort Study (MACS). In the HIV group, we asked about weight variation and individual perception on their fat distribution, before and after HIV diagnosis. Our focus was on (a) increased fat under the chin (b) increased fat on the back of the neck (c) increased abdominal girth (d) increased chest or breast fat (e) loss of fat in the face (f) loss of fat in the arms (g) loss of fat in the buttocks (h) loss of fat in the legs. The physician validated information reported by the patient after conducting a thorough physical examination. Fat maldistribution was recorded separately for fat losses and accumulations. Lipodystrophy was categorized as the presence of at least one site with moderate or severe fat loss or presence of at least one site with moderate or severe fat accumulation, excluding isolated abdominal fat accumulation. In summary, the lipodystrophy was defined by patient report (standardized questionnaire) of peripheral lipoatrophy (face, arms, buttocks or legs) with or without central fat accumulation (abdomen, dorsal-cervical fat pad). The diagnosis of central adiposity was defined by a waist-hip ratio (WHR) of > 0.90 in men and > 0.80 in women.
Laboratory analysis
Plasma and serum were obtained after an overnight fast (12 h), aliquoted and stored at – 80 °C. Plasma glucose levels; hemoglobin A1c; triglycerides; total cholesterol and fractions (LDL-chol; HDL-chol)((Labtest, Brazil) were measured as previously described [36]. Serum was also used to evaluate levels of Visfatin and Adiponectin [AdipoGen Inc, Switzerland], insulin, leptin, and adiponectin [Millipore, USA]) and lipopolysaccharides (LPS [Lonza Inc., USA]). Related to insulin determination, the lowest level of insulin that can be detected by this assay is 1 µU/mL, and the specificity of the analytical test for human insulin is 100% (ED (50) = 0.68 nM). The intra-assay variation is 5.96 ± 1.17 and the inter-assay variation is 10.3 ± 0.9 (EMD Millipore, USA cat#EZHI-14K). The homeostasis model assessment (HOMA-IR) from the formula [glucose (mmol/L) × Insulin (μU/mL)/22.5], was used to estimate insulin resistance.
Immunological data
The data referred to the immunological HIV group were obtained from medical records. Viral load (copies/ml) and T CD4+ lymphocytes number (cells/mm3) were compiled 3 months before or after the beginning of the study, as well as the antiretroviral therapy used for them.
Statistical analysis
The results were expressed as mean ± standard deviation (mean ± SD). Mann–Whitney test was used to compare the results between two groups, and for more than two groups, we used ANOVA. The Spearman correlation tests were used to check the linear relation between two variables. Statistical significance was assumed if p < 0.05 for all statistical tests. Statistical analyses were used according to SSPS v.16.0 software.
Results
The anthropometric and clinical characteristics of patients and controls are shown in Table 1. The mean age for HIV patients (40.4 ± 9.4 × 34.0 ± 12.1 years) was slightly but not significantly higher compared to controls. Body mass index (BMI) and waist to hip ratio were similar in controls and HIV patients.
Table 1.
Characteristic of study population
| Variable | Control | HIV |
|---|---|---|
| Number | 10 | 67 |
| Male sex (%) | 60 | 59.7 |
| Age (years) | 34 ± 12.1 | 40.4 ± 9.0 |
| BMI | 24.3 ± 2.8 | 22.9 ± 2.7 |
| Waist circumference (cm) | 80.1 ± 10.3 | 82.5 ± 7.0 |
| Total fat (mm) | 109.3 ± 39.1 | 89.5 ± 35.9 |
| Central fat (mm) | 78.0 ± 28.0 | 65.3 ± 25.6 |
| waist/hip | 0.8 ± 0.10 | 0.89 ± 0.07 |
In Table 2 we can observe that fasting plasma glucose in the HIV group (82 ± 7 mg/dL) was similar to controls (79 ± 11 mg/dL). Fasting serum insulin levels were modestly but significantly elevated in HIV patients (10.1 ± 5.4 × 7.0 ± 2.5 μU/mL p > 0.05) and in accordance, the HOMA index was higher in HIV patients (1.9 ± 0.9 × 1.5 ± 0.6 p < 0.05). Fasting plasma triglycerides were higher in the HIV group than in the controls (158 ± 91 × 78 ± 27 mg/dL p < 0.001). Fasting total and LDL cholesterol levels were similar in the HIV and controls. Mean HDL-C was lower in the HIV group than in the control group (50.9 ± 15.7 × 57.2 ± 12.3 mg/dL p < 0.05), and the ratio of triglycerides to HDL was also higher in HIV patients (3.8 ± 3.2 × 1.5 ± 0.7 p < 0.001). All the HIV patients were on stable HAART and plasma HIV-1 viral load was undetectable in 79% of the patients, and the T CD4+ lymphocytes number was over 350 in 95% of these patients. The levels of visfatin, leptin and adiponectin were similar between controls and HIV patients (Table 2). However, circulating levels of LPS were higher in HIV patients than in controls (0.23 ± 0.25 × 0.10 ± 0.1 EU/mL p = 0.01) (Table 2 and Fig. 1).
Table 2.
Clinical and laboratory of study population
| Variables | Control | HIV |
|---|---|---|
| Number | 10 | 67 |
| Fasting plasma glucose (mg/dL) | 79.2 ± 11 | 82.2 ± 7 |
| Triglycerides (mg/dL) | 78.8 ± 27.5 | 158.3 ± 91.2** |
| Total cholesterol (mg/dL) | 173.1 ± 19.8 | 189.0 ± 45 |
| LDL (mg/dL) | 101.4 ± 28.6 | 107.9 ± 36.08 |
| HDL (mg/dL) | 57.2 ± 12.3 | 50.9 ± 15.7* |
| Visfatin (ng/mL) | 4.5 ± 1.2 | 6.9 ± 6.2 |
| Leptin (ng/mL) | 8.7 ± 6.7 | 6.8 ± 7.5 |
| Adiponectin (μg/mL) | 9.1 ± 3.1 | 9.0 ± 9.14 |
| Trig/HDL | 1.5 ± 0.7 | 3.76 ± 3.18** |
| Insulin (μU/mL) | 7.0 ± 2.5 | 10.1 ± 5.4* |
| HOMA | 1.5 ± 0.6 | 1.9 ± 0.9* |
| LPS (EU/mL) | 0.08 ± 0.05 | 0.23 ± 0.25* |
* p < 0.05, ** p < 0.001
Fig. 1.
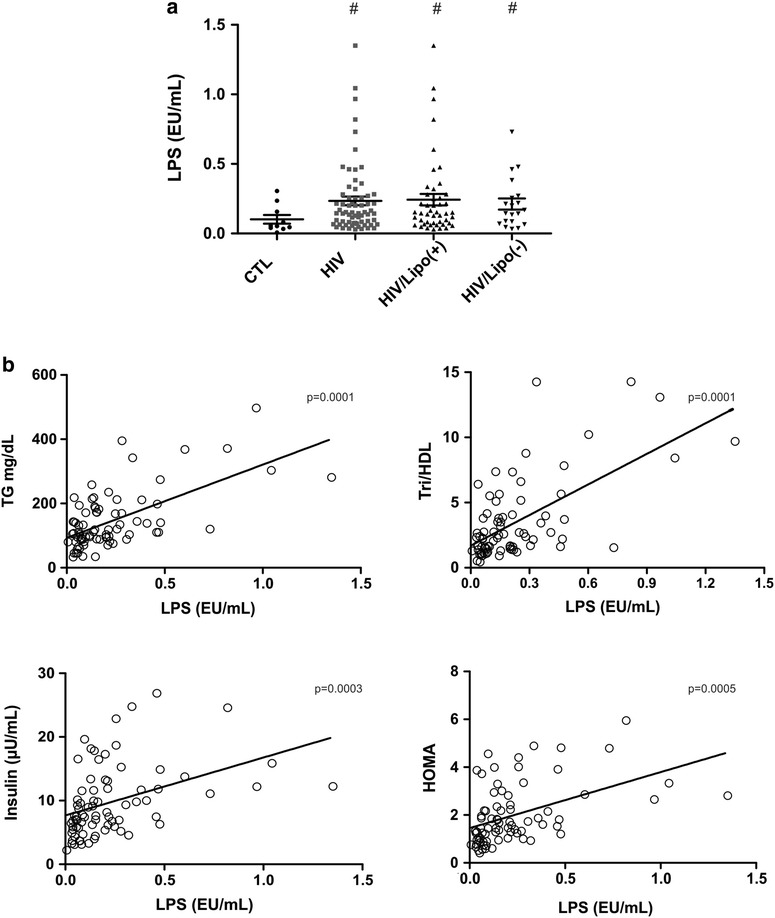
Circulating levels of LPS and Spearman correlation between circulating LPS leaves and selected insulin resistance index. a LPS circulating levels in controls (CTL), HIV patients, HIV patients with lipodystrophy (HIV/Lipo(+)) and without lipodystrophy (HIV/Lipo(−)) #p < 0.05 vs. control. b Correlation between LPS and TG (Triglycerides) (r = 0.49, p = 0.0001), between LPS and TG/HDL (r = 0.50, p = 0.0001), between LPS and insulin (r = 0.52, p = 0.0003), and between LPS and HOMA-IR (r = 0.52, p = 0.0005), in the entire group. O: HIV patients
In this regard, when we divided the HIV patients into two groups (with lipodystrophy and without lipodystrophy) the increase in LPS circulating levels were more evident in patients with lipodystrophy (0.42 ± 0.30 × 0.10 ± 0.1 EU/mL p = 0.01) than in patients without this abnormality 0.21 ± 0.16 × 0.10 ± 0.1 EU/mL p = 0.03), compared to controls in both situations. The levels of adiponectin were significantly lower in HIV patients with lipodystrophy (7.1 µg/mL × 10.1 µg/mL p < 0.02), but no differences in the levels of leptin or visfatin were observed between the groups.
Correlations
Related to the index of insulin resistance in HIV patients there was a positive correlation between insulin and TG/HDL (r = 0.64, p = 0.0001), between HOMA and TG/HDL (r = 0.61, p = 0.0001) and a negative correlation was seen between adiponectin and TG/HDL (r = − 0.50, p = 0.0011).
There was a weak inverse correlation between the number of CD4+ T cells and TG/HDL (r = 0.22 p < 0.05), and HOMA (r = 0.23 p < 0.04). However, no correlation was observed between CD4+ and LPS, but in very high LPS levels (> 0.5 EU/ml) there was a clear positive correlation between these two parameters (r = 0.94 p < 0.01).
There was a negative correlation between LPS and HDL (r = − 0.49, p = 0.0001), and a positive correlation between LPS and TG (r = 0.49, p = 0.0001), between LPS and TG/HDL (r = 0.50, p = 0.0001), between LPS and insulin (r = 0.52, p = 0.0003) and between LPS and HOMA-IR (r = 0.52, p = 0.0005), in HIV patients on HAART (Fig. 1). We found no significant correlations between BMI and total central fat or LPS levels, between LPS and adiponectin, visfatin or leptin.
Discussion
In the present study, we investigated the role of hormones produced by adipose tissue and LPS plasma levels, which is a marker of intestinal permeability, in the insulin resistance in HIV patients. Our results demonstrated an increase in LPS levels in HIV patients, suggesting a relationship between LPS levels and metabolic alterations, particularly affecting lipids and insulin resistance in these patients. On the other side, the hormones visfatin, adiponectin, and leptin did not show significant alterations in HIV patients on HAART.
Although glucose clamp is the standard gold method to measure insulin resistance, different studies have shown a close correlation between this method and HOMA for this purpose, and our data showed that HIV patients had higher levels of HOMA. Moreover, the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) concentration ratio has been shown to be a reliable index of insulin resistance, similar to the fasting serum insulin [37–40]. The TG/HDL-C ratio as a direct measure of insulin resistance in overweight or obese adults was first reported by Reaven et al. and then confirmed in a larger sample [40]. Our data showed that in HIV patients treated with HAART the TG/HDL-C ratio presented a strong correlation with measurements of insulin resistance as fasting insulin and HOMA, and a negative correlation with adiponectin, indicating that in these patients this index can be used as a measurement of reduced insulin sensitivity.
As pointed recently [41], HOMA-IR does not provide a very precise estimate of peripheral insulin action and although not completely proved it might instead measure the ability of insulin to inhibit hepatic glucose production in the fasting state. In this regard, it is interesting that previous data showed that in HIV patients there is an infection of Kupffer cells in the liver which enhanced LPS cell-surface receptors (TLR4) and increased IL-6 and TNF-a expression [42]. Taken together these results with our data showing an increase in LPS levels we can suggest that these alterations can induce a clear hepatic insulin resistance.
The previous review showed that the highest prevalence of lipodystrophy is observed in women [43], associated with lower levels of HDL-cholesterol and insulin resistance. In this regard, estrogens and estrogens receptors have critical roles in adipose differentiation and fat distribution, contributing to explain this difference. However, in our study, the number of women is smaller making a sub-analysis according to gender more difficult.
The unique aspect of our results is the higher levels of plasma LPS in HIV patients treated with HAART and the strong positive correlation between plasma LPS and measurements of insulin resistance such as fasting insulin, HOMA and the TG/HDL-C ratio. It is important to mention that plasma LPS levels were higher in HIV patients treated with HAART with and without lipodystrophy suggesting that circulating LPS may have a role in the reduced insulin sensitivity in these patients, probably in a manner independent of fat storage.
Notably, the gastrointestinal tract is the principal source of lipopolysaccharide (LPS), because of its massive bacterial load compared to other anatomical sites [44]. Also, there is a direct and strong association between plasma LPS levels and the degree of intestinal permeability, in different pathological conditions [33], including patients with HIV. This translocation of LPS into the plasma leads to activation of both innate and adaptive immune responses [34, 35]. The innate immune response initiates through the binding of LPS to Toll-like receptor 4(TLR4), which will induce the release of inflammatory cytokines, resulting in systemic inflammation [45, 46] and insulin resistance, similar to diet-induced obesity in animals and humans [33]. In mice, the subcutaneous infusion of LPS, to reach levels that simulate the translocation of gut bacterial products, has been shown to induce glucose intolerance, higher insulin levels, insulin resistance, and very interesting increase in body weight accompanied by increases in adipose tissue [47, 48]. Whether this increase in circulating levels of LPS in HIV patients on HAART is related to changes in intestinal permeability and changes in intestinal microbiota deserves further investigation.
It is important to mention that although low levels of CD4+ T cells in gut-associated lymphoid tissues may contribute to explain microbial translocation, no inverse correlation between LPS and CD4+ T cells were identified in our study. This may be a consequence of a possible activation of CD4+ cells induced by LPS [42], which may overshadow this negative correlation. In agreement with the increase in CD4+ T cell number induced by LPS, in HIV patients on HARRT with LPS levels higher than 0.5 EU/mL, there was a positive correlation between these parameters.
As previously demonstrated HIV patients on HAART presented dyslipidemia characterized by high triglyceride levels and reduced HDL-cholesterol levels [49]. This result is by previous data that showed that in patients with HIV in use of HAART the most prevalent metabolic alterations were reduced HDL-cholesterol and hypertriglyceridemia [4]. The association of dyslipidemia with high levels of LPS may contribute to increasing the cardiovascular risk in HIV patients on HAART. Previous data showed that increased levels of LPS are associated with endothelial dysfunction in HIV-infected patients [50] and adverse metabolic outcomes [33, 45, 47]. Also, plasma levels of LPS were also associated with progression of atherosclerosis in HIV patients treated with HAART [49] associated with a negative correlation between large HDL particles measured by NMR spectroscopy and LPS in these patients. This negative association is of concern because greater levels of large HDL particles are associated with a reduced risk of CVD and insulin resistance [51], and lower levels of large HDL are associated with endothelial dysfunction and coronary artery disease [52]. It is important to mention that in the SMART study HDL was associated with a decreased risk of coronary artery disease in HIV-patients [53]. Together, this raises the possibility that an increase in LPS levels during prolonged ART may contribute to the increased CVD risk in part due to adverse effects on HDL particles.
There is some weakness in our study that deserves comments: First we correlate LPS circulating levels with insulin resistance, but we did not use the standard gold method to measure insulin resistance as described above. The euglycemic hyperinsulinemic glucose clamp is a relatively complex method and difficult to perform in the number of patients that we studied. Also, at the time we started this study we did not have a methodology to investigate the microbiota of the patients nor the circulating levels of short chain fatty acids (SCFA), which would have allowed us to have better correlations between the changes in microbiota/intestinal permeability with LPS and SCFA.
In summary, our results showed an increase in LPS levels in HIV patients using HAART, and a correlation between plasma LPS and markers of insulin resistance, suggesting a relationship between LPS levels and metabolic alterations, mainly affecting lipids and insulin resistance in these patients.
Authors’ contributions
MNP, DOM, DG, AS, MJAS researched data, contributed to discussion, wrote/reviewed/edited manuscript. ISS researched data. RJP reviewed/edited manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank L. Janeri, in memorium (Department of Internal Medicine, UNICAMP, Campinas, São Paulo) and J. Pinheiro (Department of Internal Medicine, UNICAMP, Campinas, São Paulo) for their technical assistance.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee local (No. 124/2007-FCM/Unicamp) and all participants received informed consent after approval by Ethics Committee.
Funding
We also acknowledge the financial support CEPID/Fapesp 201307607-8, OCRC (Obesity and Comorbidities Research Center), INCT (National Institute of Science and Technology for Diabetes and Obesity) 573856/2008-7 and CAPES/CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- HIV
human immunodeficiency virus
- HAART
highly active antiretroviral therapy
- IR
insulin resistance
- TNF-α
tumor necrosis factor alpha
- IL6
interleukin 6
- LPS
lipopolysaccharide
- BMI
body mass index
- WHR
waist-hip ratio
- HOMA
Homeostatic model assessment
- T CD4+
T-helper cells (cluster of differentiation 4)
- TG
triglycerides
- TLR4
Toll-like receptor 4
- ART
antiretroviral therapy
- CVD
cardiovascular disease
Contributor Information
Marcelo Nardi Pedro, Email: dioze@fcm.unicamp.br.
Daniela Oliveira Magro, Email: danimagro@terra.com.br.
Elizabete Urbano Pinaço Pinto da Silva, Email: nutri.elizabeteurbano@gmail.com.
Dioze Guadagnini, Email: diozeg@gmail.com.
Andrey Santos, Email: andreysts@gmail.com.
Rogerio de Jesus Pedro, Email: rjpedro.res@gmail.com.
Mario José Abdalla Saad, Phone: +55 19 35218950, Email: msaad@fcm.unicamp.br.
References
- 1.Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A, Wasmuth JC, van Sighem A, Kirk O, Obel N, Panos G, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥ 500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41:433–445. doi: 10.1093/ije/dyr164. [DOI] [PubMed] [Google Scholar]
- 2.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 3.Tiozzo E, Konefal J, Adwan S, Martinez LA, Villabona J, Lopez J, Cutrono S, Mehdi SM, Rodriguez A, Woolger JM, Lewis JE. A cross-sectional assessment of metabolic syndrome in HIV-infected people of low socio-economic status receiving antiretroviral therapy. Diabetol Metab Syndr. 2015;7:15. doi: 10.1186/s13098-015-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beraldo RA, Santos APD, Guimarães MP, Vassimon HS, Paula FJA, Machado DRL, Foss-Freitas MC, Navarro AM. Body fat redistribution and changes in lipid and glucose metabolism in people living with HIV/AIDS. Rev Bras Epidemiol. 2017;20:526–536. doi: 10.1590/1980-5497201700030014. [DOI] [PubMed] [Google Scholar]
- 5.Calmy A, Hirschel B, Cooper DA, Carr A. Clinical update: adverse effects of antiretroviral therapy. Lancet. 2007;370:12–14. doi: 10.1016/S0140-6736(07)61027-7. [DOI] [PubMed] [Google Scholar]
- 6.Calza L, Manfredi R, Chiodo F. Insulin resistance and diabetes mellitus in HIV-infected patients receiving antiretroviral therapy. Metab Syndr Relat Disord. 2004;2:241–250. doi: 10.1089/met.2004.2.241. [DOI] [PubMed] [Google Scholar]
- 7.Mbunkah HA, Meriki HD, Kukwah AT, Nfor O, Nkuo-Akenji T. Prevalence of metabolic syndrome in human immunodeficiency virus-infected patients from the South-West region of Cameroon, using the adult treatment panel III criteria. Diabetol Metab Syndr. 2014;6:92. doi: 10.1186/1758-5996-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visnegarwala F, Chen L, Raghavan S, Tedaldi E. Prevalence of diabetes mellitus and dyslipidemia among antiretroviral naive patients co-infected with hepatitis C virus (HCV) and HIV-1 compared to patients without co-infection. J Infect. 2005;50:331–337. doi: 10.1016/j.jinf.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Salmon-Ceron D, Protopopescu C, Leport C, Raffi F, Chene G. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. Aids. 2012;26:303–314. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 10.Guillen MA, Mejia FA, Villena J, Turin CG, Carcamo CP, Ticse R. Insulin resistance by homeostasis model assessment in HIV-infected patients on highly active antiretroviral therapy: cross-sectional study. Diabetol Metab Syndr. 2015;7:49. doi: 10.1186/s13098-015-0046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arama V, Tiliscan C, Streinu-Cercel A, Ion D, Mihailescu R, Munteanu D, Hristea A, Arama SS. Insulin resistance and adipokines serum levels in a caucasian cohort of HIV-positive patients undergoing antiretroviral therapy: a cross sectional study. BMC Endocr Disord. 2013;13:4. doi: 10.1186/1472-6823-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boufassa F, Goujard C, Viard JP, Carlier R, Lefebvre B, Yeni P, Bouchaud O, Capeau J, Meyer L, Vigouroux C. Immune deficiency could be an early risk factor for altered insulin sensitivity in antiretroviral-naive HIV-1-infected patients: the ANRS COPANA cohort. Antivir Ther. 2012;17:91–100. doi: 10.3851/IMP1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addy CL, Gavrila A, Tsiodras S, Brodovicz K, Karchmer AW, Mantzoros CS. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab. 2003;88:627–636. doi: 10.1210/jc.2002-020795. [DOI] [PubMed] [Google Scholar]
- 14.Estrada V, Martinez-Larrad MT, Gonzalez-Sanchez JL, de Villar NG, Zabena C, Fernandez C, Serrano-Rios M. Lipodystrophy and metabolic syndrome in HIV-infected patients treated with antiretroviral therapy. Metabolism. 2006;55:940–945. doi: 10.1016/j.metabol.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney LL, Brennan AM, Mantzoros CS. The role of adipokines in relation to HIV lipodystrophy. Aids. 2007;21:895–904. doi: 10.1097/QAD.0b013e3280adc91e. [DOI] [PubMed] [Google Scholar]
- 16.Leow MK, Addy CL, Mantzoros CS. Clinical review 159: human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. J Clin Endocrinol Metab. 2003;88:1961–1976. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 17.Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7:137–150. doi: 10.1038/nrendo.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitas P, Carvalho D, Santos AC, Mesquita J, Matos MJ, Madureira AJ, Martinez E, Sarmento A, Medina JL. Lipodystrophy defined by Fat Mass Ratio in HIV-infected patients is associated with a high prevalence of glucose disturbances and insulin resistance. BMC Infect Dis. 2012;12:180. doi: 10.1186/1471-2334-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlay JC, Visnegarwala F, Bartsch G, Wang J, Peng G, El-Sadr WM, Gibert C, Kotler D, Grunfeld C, Raghavan S. Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs. abacavir and lamivudine. J Acquir Immune Defic Syndr. 2005;38:147–155. doi: 10.1097/01.qai.0000143599.64234.15. [DOI] [PubMed] [Google Scholar]
- 20.Magkos F, Mantzoros CS. Body fat redistribution and metabolic abnormalities in HIV-infected patients on highly active antiretroviral therapy: novel insights into pathophysiology and emerging opportunities for treatment. Metabolism. 2011;60:749–753. doi: 10.1016/j.metabol.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;3:2. doi: 10.1186/1758-5996-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 23.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36:245–271. doi: 10.1210/er.2014-1100. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 28.Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–393. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrer M, Ruiz A, Lanza F, Haange SB, Oberbach A, Till H, Bargiela R, Campoy C, Segura MT, Richter M, et al. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ Microbiol. 2013;15:211–226. doi: 10.1111/j.1462-2920.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- 30.Caricilli AM, Saad MJ. Gut microbiota composition and its effects on obesity and insulin resistance. Curr Opin Clin Nutr Metab Care. 2014;17:312–318. doi: 10.1097/MCO.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho BM, Saad MJ. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediat Inflamm. 2013;2013:986734. doi: 10.1155/2013/986734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 33.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 34.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 35.Bukh AR, Melchjorsen J, Offersen R, Jensen JM, Toft L, Stovring H, Ostergaard L, Tolstrup M, Sogaard OS. Endotoxemia is associated with altered innate and adaptive immune responses in untreated HIV-1 infected individuals. PLoS ONE. 2011;6:e21275. doi: 10.1371/journal.pone.0021275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carvalho BM, Oliveira AG, Ueno M, Araújo TG, Guadagnini D, Carvalho-Filho MA, Geloneze B, Lima MM, Pareja JC, Carvalheira JB, Saad MJ. Modulation of double-stranded RNA-activated protein kinase in insulin sensitive tissues of obese humans. Obesity. 2013;21:2452–2457. doi: 10.1002/oby.20410. [DOI] [PubMed] [Google Scholar]
- 37.Haugaard SB, Andersen O, Hansen BR, Andersen UB, Volund A, Iversen J, Nielsen JO, Madsbad S. In nondiabetic, human immunodeficiency virus-infected patients with lipodystrophy, hepatic insulin extraction and posthepatic insulin clearance rate are decreased in proportion to insulin resistance. Metabolism. 2005;54:171–179. doi: 10.1016/j.metabol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Chu JW, Abbasi F, Beatty GW, Khalili M, Koch J, Rosen A, Schmidt JM, Stansell JD, Reaven GM. Methods for quantifying insulin resistance in human immunodeficiency virus-positive patients. Metabolism. 2003;52:858–861. doi: 10.1016/S0026-0495(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 41.Reaven GM. What do we learn from measurements of HOMA-IR? Diabetologia. 2013;56:1867–1868. doi: 10.1007/s00125-013-2948-3. [DOI] [PubMed] [Google Scholar]
- 42.Mosoian A, Zhang L, Hong F, Cunyat F, Rahman A, Bhalla R, Panchal A, Saiman Y, Fiel MI, Florman S, et al. Frontline Science: HIV infection of Kupffer cells results in an amplified proinflammatory response to LPS. J Leukoc Biol. 2017;101:1083–1090. doi: 10.1189/jlb.3HI0516-242R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naidu S, Ponnampalvanar S, Kamaruzzaman SB, Kamarulzaman A. Prevalence of metabolic syndrome among people living with HIV in developing countries: a systematic review. AIDS Patient Care STDS. 2017;31:1–13. doi: 10.1089/apc.2016.0140. [DOI] [PubMed] [Google Scholar]
- 44.Kelly CJ, Colgan SP, Frank DN. Of microbes and meals: the health consequences of dietary endotoxemia. Nutr Clin Pract. 2012;27:215–225. doi: 10.1177/0884533611434934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clement K, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33:1925–1932. doi: 10.2337/dc10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaffler A, Scholmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010;31:228–235. doi: 10.1016/j.it.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Cani PDAJ, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 48.Creely SJ, McTernan PG, Kusminski CM, Fisher FM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 49.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blodget E, Shen C, Aldrovandi G, Rollie A, Gupta SK, Stein JH, Dube MP. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS ONE. 2012;7:e42624. doi: 10.1371/journal.pone.0042624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ford MA, McConnell JP, Lavi S, Rihal CS, Prasad A, Sandhu GS, Hartman SJ, Lerman LO, Lerman A. Coronary artery endothelial dysfunction is positively correlated with low density lipoprotein and inversely correlated with high density lipoprotein subclass particles measured by nuclear magnetic resonance spectroscopy. Atherosclerosis. 2009;207:111–115. doi: 10.1016/j.atherosclerosis.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, Neuhaus J, Paton NI, Friis-Moller N, Lampe F, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–529. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


