Fig. 1.
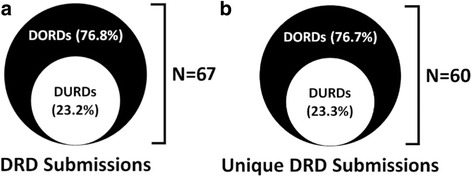
a Drug for rare diseases submissions, including resubmissions of drugs with same indication due to the availability of new evidence that may change the original recommendation. b Unique submissions of drugs for rare diseases, where we considered only the latest submission of drugs with multiple submissions for the same indication
