Abstract
In the past, the horizontal transfer of antimicrobial resistance genes was mainly associated with conjugative plasmids or transposons, whereas transduction by bacteriophages was thought to be a rare event. In order to analyze the likelihood of transduction of antimicrobial resistance in the field of clinical veterinary medicine, we isolated phages from Escherichia coli from a surgery suite of an equine clinic. In a pilot study, the surgery suite of a horse clinic was sampled directly after surgery and subsequently sampled after cleaning and disinfection following a sampling plan based on hygiene, surgery, and anesthesia. In total, 31 surface sampling sites were defined and sampled. At 24 of these 31 surface sampling sites, coliphages were isolated. At 12 sites, coliphages were found after cleaning and disinfection. Randomly selected phages were tested for their ability of antimicrobial resistance transduction. Ten of 31 phages were detected to transfer antimicrobial resistance. These phages most often transduced resistance to streptomycin, encoded by the addA1 gene (n = 9), followed by resistance to chloramphenicol by cmlA (n = 3) and ampicillin (n = 1). This is, to the best of our knowledge, the first report on antimicrobial resistance-transferring bacteriophages that have been isolated at equine veterinary clinics.
Keywords: Escherichia coli, phage, antimicrobial resistance, surgery, equine
Introduction
Nosocomial infections in horse clinics, caused by multi-resistant pathogens, have dramatically increased. Hence, action must be taken to ensure safety and infection control, e.g., the implementation of targeted prevention programs, including cleaning and disinfection [1]. The incidence of infections at surgery sites is frequently a cause of morbidity, and Escherichia coli is one of these relevant pathogens [2]. Next to the constitution of the patient and to surgery techniques, surgery room contamination has been defined as an important risk factor for E. coli nosocomial infection [2, 3]. For treating these nosocomial infections, multi-resistant E. coli can be a major problem [2, 4–6]. For example, in recent years, the increase of extended-spectrum ß-lactamase (ESBL)-producing E. coli, isolated from horses, has been of importance [4]. Antimicrobial resistance in bacteria is in general a global and growing threat to public health. This emphasizes the need for monitoring of resistance, as well as highlights the need for responsible antimicrobial stewardship and appropriate antimicrobial usage [7]. Next to a clonal spread of resistant bacteria, the horizontal transmission of antimicrobial resistance genes is of upper importance and has been associated mainly with conjugative plasmids or transposons, and only to a minor extent to transduction by bacteriophages [8, 9].
Nevertheless, recent studies show that bacteriophages may play a significant role in horizontal gene transfer in nature [10, 11]. Moreover, the frequent presence of antimicrobial resistance-transferring phages on food has already been documented [12, 13].
Nonetheless, there are high expectations from phage therapy to defeat multi-resistant pathogens and from the use of phage cocktails in prophylaxis [14, 15]. A possible transduction of antimicrobial resistance genes by these phages has not been a matter of concern in medicine so far. To analyze the possibility of antimicrobial resistance transduction by phages in equine surgery suites, we investigated the occurrence of bacteriophages at selected sampling sites and we determined their potential to transduce antimicrobial resistance.
Materials and methods
Sample collection and preparation
In 2016, sampling sites were defined by previous inspections with an on-site team of experts in hygiene, surgery, and anesthesia (Table 2).
Table 2.
Sampling sites, total number of isolated phages, and transduction of antimicrobial resistance
| Category | Sampling site | Total number of detected phages | Number of analyzed phages | Antimicrobial resistance transduced by phages |
|---|---|---|---|---|
| Site with patient contact | Respiration apparatus | 0 | 0 | |
| Surgical table surface | >10* | 1 | StrR, AmpR | |
| Surgical table leg | 1–5* | 1 | ||
| Surgical table edges | >10† | 1 | ||
| Hobbles | >10‡ | 2 | ||
| Hobble fixers | 0 | 0 | ||
| Surgical gloves | 0 | 0 | ||
| Site with human contact | Respiration apparatus monitor frame | 0 | 0 | |
| Crane remote control | 1–5* | 1 | ||
| Anesthesia unit drawer | >10‡ | 2 | ||
| Surgical light grasp | 0 | 0 | ||
| Surgical light switch | 1–5* | 1 | ||
| Chair seat | 1–5† | 1 | ||
| Soap dispenser | 1–5* | 1 | ||
| Door handle to recovery room | 0 | 0 | ||
| Main-door handle outside | 1–5* | 1 | StrR | |
| Main-door handle inside | 1–5* | 1 | StrR | |
| Difficult to clean | Drain on floor | >10‡ | 2 | StrR |
| Control panel for electronic equipment | >10‡ | 2 | ||
| Venetians opener | 1–5* | 1 | ||
| Particulate air filter | >10‡ | 2 | ||
| Surfaces | Floor in front of washing room | 1--5* | 1 | StrR, CmR |
| Floor under surgery table | 1–5* | 1 | StrR | |
| Floor in front of respiration apparatus | >10* | 1 | StrR | |
| Floor in front of entrance door | 1--5‡ | 2 | CmR | |
| Surface of recovery room door | 1--5‡ | 2 | StrR | |
| Surface of respiration apparatus | 1--5† | 1 | ||
| Table legs of preparation table | 1--5† | 1 | ||
| Surface of preparation table | 1--5† | 1 | StrR, CmR | |
| Wall over preparation table | 1–5* | 1 | ||
| Surgical light surface | 0 | 0 |
*Before
†Afer
‡Before and after cleaning and disinfection
Within 10 min following patient removal from the surgery suit, 31 surface samples from these sites were collected, using sterile swabs or sponges (Medical Wire & Equipment, Corsham, Wiltshire, England). Figure 1 shows an overview of the surgery room, with important sampling sites.
Fig. 1.

Overview of the equine surgery suite, where samples were taken. Important sampling sites are tagged by circles with numbers. 1, Respiration apparatus; 2, surgery table surface; 3, drain on floor; 4, floor under surgery table; 5, floor in front of respiratory apparatus; 6, floor in front of entrance door
A second batch of 31 samples was collected from the same sampling sites after cleaning and disinfection. Each swab and sponge was separately sampled in a sterile plastic jar and overlaid with 2 ml sterile NaCl (0.9%) and sponges with 10 ml sterile NaCl (0.9%).
After manual shaking and kneading, the sample solutions were used to isolate bacteriophages.
Isolation of bacteriophages and preparation of lysates
Three milliliters of ssMSA (semi solid Modified Scholtens’ Agar, prepared as given in ISO 10705 [16]) with 18 µ1 CaCl2 was heated up to 45 °C and kept liquid. E. coli ATCC 13706 was grown to an optical density of McFarland 0.5. After adding 1 ml of this culture and 1 ml of the sample solution (prepared as described under sample collection and preparation), the suspension was vortexed and overlaid on a pre-warmed MSA (semi-solid Modified Scholtens’ Agar) plate. After incubation of 15 h at 37 °C overnight, the plates were evaluated for visible plaques (Fig. 2). Up to five single plaques were isolated (randomly) using a sterile pipette tip, transferred into a tube with 300 µl MSB (Modified Scholtens’ Broth), and the gel was manually cracked into small pieces. These tubes were incubated at 30 °C for 90 min. The lysate was filtered through a sterile 0.2 µm filter which was pre-moistened with MSB. Chloroform was added to a final concentration of 5% v/v, and the lysate was stored at 4 °C.
Fig. 2.
A plaque isolation plate, as described in section Materials and methods with typical plaques visible on host strain ATCC 13706
Cleaning and disinfection procedure at sampling sites
In this study, surgery rooms were cleaned and disinfected after each surgery with the cleaning agent Hexaquart® plus (B. Braun Melsungen AG, Melsungen, Germany). The active ingredients of this surface disinfectant are didecyldimethylammonium chloride and N, N-bis(3-aminopropyl) dodecylamine. The detergent is used in accordance with the manufacturer’s instructions as a 2% solution (sprayed directly onto all surfaces as foam and, after an exposure time of 15 to 20 min, rinsed with water). Some sensitive areas, such as hobbles or air filters, cannot be cleaned and are replaced at regular intervals.
Transduction
From every sample point, one phage was tested for antimicrobial resistance transduction into ATCC 13706. Transduced bacteria were selected against resistance to ampicillin (35 µg/ml AmpR), tetracycline (20 µg/ml TetR), streptomycin (30 µg/ml StrR), and chloramphenicol (35 µg/ml CmR). Transduction was performed as previously described [12]. In brief, an overnight culture from E. coli ATCC 13706 was grown in 30 ml LB (Lysogeny broth) media containing 5 mM CaCl2 and 100 mM MgSO4. A dilution of 1:100 in the same media was grown to an optical density of 0.8 at 600 nm, 8 ml was centrifuged at 6000 g, and the pellet was dissolved by vortexing in 2 ml of LB containing 5 mM CaCl2 and 100 mM MgSO4; 750 µl was added to 40 µl of the lysate (preparation is described under phage isolation and preparation above) and incubated at 37 °C. After 30 min, 800 µl of trisodium citrate (pH 5.5) was added. After homogenization, 4 ml of LB media was added and the batch was incubated again at 37 °C while shaking it. After centrifugation of the cells at 6000 g for 5 min, the supernatant was removed, except 400 µl. An amount of 100 µl of the resuspended pellet was streaked on LB plates containing the respective antimicrobial substance. After incubation at 37 °C overnight, colonies were selected for antimicrobial resistance testing.
Identification of resistance genes
Resistance genes in transduced E. coli isolates were detected by PCR, using primers given in Table 1. DNA was extracted using a DNA Mini Kit (Qiagen GmbH, Hilden, Germany). The PCRs were carried out in a 25-µl reaction mixture containing 30 ng DNA, 12.5 µl Kapa2G polymerase from a Fast hot start kit (Kapa Biosystems, Wilmington, MA, USA), 2 µl MgCl2 (25 mM), and 0.25 µl each primer (100 mM).
Table 1.
Primers used for detection of antimicrobial resistance genes
| Gen | Primer forward | Primer reverse | TA (°C) | Ref. |
|---|---|---|---|---|
| strA | 5′ CCAATCGCAGATAGAAGGCAAG 3′ | 5′ ATCAACTGGCAGGAGGAACAGG 3′ | 65 | [22] |
| addA1 | 5′ AACGACCTTTTGGAAACTTCGG 3′ | 5′ TTCGCTCATCGCCAGCCCAG 3′ | 61 | [22] |
| blaTEM | 5′ CAGCGGTAAGATCCTTGAGA 3′ | 5′ ACTCCCCGTCGTGTAGATAA 3′ | 55 | [23] |
| cmlA | 5′ CCGCCACGGTGTTGTTGTTATC 3′ | 5′ CACCTTGCCTGCCCATCATTAG 3′ | 54 | [24] |
Characterization of coliphage
The DNA of a single phage was extracted by phenol/chloroform using a titered (109 pfu/ml) lysate, as described previously, but without using a caesium chloride gradient centrifugation [17]. The DNA was quantified and restricted with enzyme PvuII (Roche Diagnostics GmbH, Vienna, Austria), a rare cutter, using manufacturer’s protocol and separated on a 1% agarose gel.
Minimal inhibitory concentration (MIC)
With antimicrobial-resistant transduced bacteria obtained, MICs were carried out to determine the degree of resistance, using microdilution procedure and epidemiological cut-offs defined by EUCAST [18]. For this purpose, the transduced bacterial isolates were incubated in Müller Hinton nutrient broth, with different concentrations (2 mg/1 to 256 mg/1) of the respective antimicrobial substance for 24 h at 35 °C.
Results
Resistance-transducing phages isolated from equine surgery units
Coliphages were detected at 19 sampling sites after surgery and at 12 sampling sites after cleaning and disinfection respectively. Of 31 isolated and tested phages, 10 were able to transduce one or more of the tested antimicrobial resistances (TetR, AmpR, StrR, CmR) to E. coli ATCC 13706. These antimicrobial substances were selected based on lead substances, stability in the environment, treatment options, and resistance situation in Austria. Thus, a total of 32% of all phages could transduce one or more antimicrobial resistances. Resistance to streptomycin was transduced most frequently (n = 9). Three phages were able to transduce resistance against chloramphenicol. Two of these phages transduced chloramphenicol and streptomycin resistance. Another phage transduced ampicillin resistance as well as streptomycin resistance. Isolation sites of phages and antimicrobial resistance-transducing phages can be seen in Table 2. Antimicrobial resistance-transducing phages were found at seven locations before cleaning and disinfection (at the main-door handle outside and inside, in the drain, on the floor under the surgery table, in front of the washing room, and in front of the respiration apparatus, on the surgery table surface) and at three locations after cleaning and disinfection (on the floor in front of the entrance door, on the surface of the recovery room door, and on the surface of the preparation table).
Coliphage characterization
As this was a pilot study, the antimicrobial resistance-transducing phages were only briefly characterized via an enzymatic restriction using PvuII. In Fig. 3, three phages isolated from one sampling site have been analyzed. Two profiles gave the same restriction pattern, whereas one phage differed from the others.
Fig. 3.
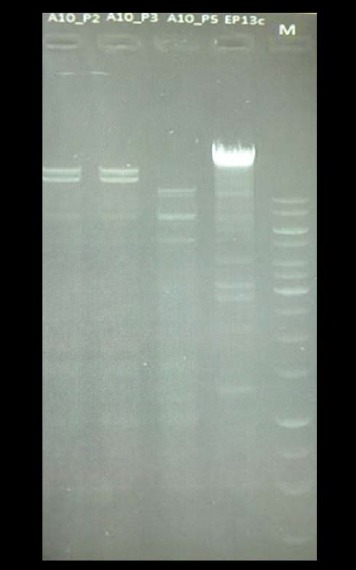
Restriction with PvuII of DNA from three different E. coli phage isolates from one sampling site, drain on floor (Table 2). From left to right: slot 1, phage number 2; slot 2, phage number 3; slot 3, phage number 5; slot 4, control phage; slot 5, molecular weight marker. Phage numbers 2 and 3 gave the same restriction pattern, and phage number 5 differs from those two
Detection of resistance genes in transduced E. coli
The addA1 gene fragment could be amplified in five transduced streptomycin-resistant E. coli isolates. The cmlA fragment was found in one chloramphenicol-resistant E. coli isolate. For all other isolates, no resistance gene was detected by PCR.
Minimal inhibitory concentration
All transduced E. coli were analyzed for their minimal inhibitory concentration to selected antimicrobial substances. Streptomycin-resistant isolates derived a minimal inhibitory concentration of 256 mg/1 or higher. The chloramphenicol-resistant E. coli with a positive PCR for cmlA showed a MIC of 256 mg/l and the ampicillin-resistant isolate displayed a MIC of 64 mg/l.
Discussion
Nosocomial infections and surgery site infections in horse are frequently seen to be caused by E. coli as a pathogen. Isgren et al. [2] identified E. coli in 59.5% of surgery site infections. These pathogens are often multi-resistant against commonly used antimicrobial therapeutics. Surgery suite contamination by this pathogen is relevant, but strategies in cleaning and disinfection of surgery rooms are not always effective to limit or reduce the high incidence of cases, even though prevention programs may have been implemented [1]. The spread of antimicrobial resistance in this pathogen is especially of high concern. Bacteria can have either a natural (intrinsic) resistance to antimicrobial substances, or they can acquire resistance by mutation or gene transfer. Here, we show that transduction by phages can play a role in the distribution of antimicrobial resistance genes in bacteria in clinical veterinary medicine. The isolation of antimicrobial resistance transducing phage, both before and after cleaning and disinfection at critical points in a surgery suite, provides evidence that phages might be a risk for horizontal antimicrobial resistance gene transfer. Prior to cleaning, at 19 of 31 surface sampling sites, E. coli phages were isolated and after cleaning and disinfection, phages from 12 sampling sites could be isolated. Animal clinics are always places with high selection pressure for the spread of antimicrobial-resistant bacteria because of an imminent pressure caused by the use of antimicrobial substances and by patients harboring and spreading these bacteria [7, 8, 19]. Cleaning and disinfection are attributed to primarily eliminate bacteria [20], but whether these measures are effective in eliminating viruses, like bacteriophages, is not defined for most regimes. The resistance of phages to disinfectants has not yet been explored in detail, since transduction has been considered to be of little importance for the spread of antimicrobial resistance and phage has no pathogenic effect on eukaryotes. In preliminary experiments that have already been carried out, it became obvious that certain phages can survive a variety of different disinfectants, e.g., they are highly stable to alcoholic agents such as 70% ethanol, but are greatly reduced by 0.28% sodium hypochlorite [21]. The results of this work show, for the first time, that phages, able to transduce antimicrobial resistance, can be isolated in the clinical environment. Bacteriophages of E. coli can be isolated from 50% of all sampling sites in a clinical surgery room and that 32% of the phages isolated and analyzed were able to transduce antimicrobial resistance against one or more of the four antimicrobial substances tested. In previously published data on transduction of antimicrobial resistance of coliphages found on food, phages from this clinical environment were able to transduce resistance to a comparable percentage (24.7% of coliphages of poultry meat transduced antimicrobial resistance) [12]. According to the results of these experiments, it is not sufficient to restrict cleaning and disinfection to bacteria, but incorporate viruses (phages) into cleaning regimes to reduce or overcome the spread of antimicrobial resistance in clinical veterinary medicine.
According to the results of this study, transduction via bacteriophages should not be neglected for the spread of antimicrobial resistance. Obviously, bacteriophages may not always be reduced with standard surface disinfecting agents. To ensure the effective use of antimicrobial substances, more data have to be assessed about transduction of antimicrobial resistance in clinical environments to find useful strategies to fight the spread of antimicrobial resistance, e.g., to use appropriate disinfectants with virucidal activity in critical areas, such as surgery suites and clinics.
Acknowledgements
The authors thank Dr. Dmijtri Sofka for technical assistance.
Footnotes
Funding sources
This work was in part supported by the NFB (Niederoesterreichische Forschungs- und Bildungsgesellschaft) grant number LS14–006.
Authors’ contribution
M.H., I.C., and F.H. conducted practical work; F.H., M.H., and U.A. compiled the study concept and design; F.H. and M.H. prepared the article; U.A., F.H., M.H., and I.C. discussed and finished the final article; and F.H. obtained funding.
Conflict of interest
The authors declare no conflict of interests.
References
- 1.Walther B, Janssen T, Gehlen H, Vincze S, Borchers K, Wieler L, Barton A, Lübke-Becker A: Infection control and hygiene management in equine hospitals. Berl Munch Tierarztl Wochenschr 127, 486–497 (2014) [PubMed] [Google Scholar]
- 2.Isgren CM, Salem SE, Archer DC, Worsman FCF, Townsend NB: Risk factors for surgical site infection following laparotomy: Effect of season and perioperative variables and reporting of bacterial isolates in 287 horses. Equine Vet J 49, 39–44 (2017) [DOI] [PubMed] [Google Scholar]
- 3.Galuppo LD, Pascoe JR, Jang SS, Willits NH, Greenman SL: Evaluation of iodophor skin preparation techniques and factors influencing drainage from ventral midline incisions in horses. J Am Vet Med Assoc 215, 963–969 (1999) [PubMed] [Google Scholar]
- 4.Walther B, Lübke-Becker A, Stamm I, Gehlen H, Barton A, Janssen T, Wieler L, Guenther S: Suspected nosocomial infections with multi-drug resistant E. coli, including extended-spectrum beta-lactamase (ESBL)-producing strains, in an equine clinic. Berl Munch Tierarztl Wochenschr 127, 421–427 (2014) [PubMed] [Google Scholar]
- 5.Ahmed MO, Williams NJ, Clegg PD, van Velkinburgh JC, Baptiste KE, Bennett M: Analysis of risk factors associated with antibiotic-resistant Escherichia coli. Microb Drug Resist 18, 161–168 (2012) [DOI] [PubMed] [Google Scholar]
- 6.Racklyeft D, Love D: Bacterial infection of the lower respiratory tract in 34 horses. Aust Vet J 78, 549–559 (2000) [DOI] [PubMed] [Google Scholar]
- 7.Johns IC, Adams EL: Trends in antimicrobial resistance in equine bacterial isolates: 1999–2012. Vet Rec 176, 334 (2015) [DOI] [PubMed] [Google Scholar]
- 8.Boerlin P, Reid-Smith RJ: Antimicrobial resistance: its emergence and transmission. Anim Health Res Rev 9, 115–126 (2008) [DOI] [PubMed] [Google Scholar]
- 9.Normark BH, Normark S: Evolution and spread of antibiotic resistance. J Intern Med 252, 91–106 (2002) [DOI] [PubMed] [Google Scholar]
- 10.Kenzaka T, Tani K, Nasu M: High-frequency phage-mediated gene transfer in freshwater environments determined at single-cell level. ISME J 4, 648–659 (2010) [DOI] [PubMed] [Google Scholar]
- 11.Muniesa M, Colomer-Lluch M, Jofre J: Could bacteriophages transfer antibiotic resistance genes from environmental bacteria to human-body associated bacterial populations? Mob Genet Elements 3, e25847 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shousha A, Awaiwanont N, Sofka D, Smulders FJ, Paulsen P, Szostak MP, Humphrey T, Hilbert F: Bacteriophages isolated from chicken meat and the horizontal transfer of antimicrobial resistance genes. Appl Environ Microbiol 81, 4600–4606 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soucy SM, Huang J, Gogarten JP: Horizontal gene transfer: building the web of life. Nat Rev Genetics 16, 472–482 (2015) [DOI] [PubMed] [Google Scholar]
- 14.Furusawa T, Iwano H, Hiyashimizu Y, Matsubara K, Higuchi H, Nagahata H, Niwa H, Katayama Y, Kinoshita Y, Hagiwara K, Iwasaki T, Tanji Y, Yokota H, Tamura Y: Phage therapy is effective in a mouse model of bacterial equine keratitis. Appl Environ Microbiol 82, 5332–5339 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM: Phage treatment of human infections. Bacteriophage 1, 66–85 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ISO, N. ISO 10705–2 (2000): Water quality – detection and enumeration of bacteriophages Part 2: enumeration of somatic coliphages, 11–12. [Google Scholar]
- 17.Sullivan MB, Coleman ML, Weigele P, Rohwer F, Chisholm SW: Three Prochlorococcus cyanophage genomes: signature features and ecological interpretations. PLoS Biol 3, s144 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ISO, N ISO 20776–1 (2006): Clinical laboratory testing and in vitro diagnostic test systems – Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. – Part 1 Reference method for testing the invitro acitivity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. [Google Scholar]
- 19.Williams A, Christley RM, McKane SA, Roberts VL, Clegg PD, Williams NJ: Antimicrobial resistance changes in enteric Escherichia coli of horses during hospitalisation: resistance profiling of isolates. Vet J 195, 121–126 (2013) [DOI] [PubMed] [Google Scholar]
- 20.Langsrud S, Sundheim G, Borgmann-Strahsen R: Intrinsic and acquired resistance to quaternary ammonium compounds in food-related Pseudomonas spp. J Appl Microbiol 95, 874–882 (2003) [DOI] [PubMed] [Google Scholar]
- 21.Shousha A, Paulsen P, Sofka D, Hilbert M, Dinhopl N, Hilbert F: Tenazität von antibiotikaresistenzübertragenden Bakteriophagen. 16. Fachtagung für Fleisch- und Geflügelfleischhygiene. 1.-2. März, Berlin: (2016) [Google Scholar]
- 22.Maidhof H, Guerra B, Abbas S, Elsheikha HM, Whittam TS, Beutin L: A multiresistant clone of shiga toxin-producing Escherichia coli O118:(H16) is spread in cattle and humans over different European countries. Appl Environ Microbiol 68, 5834–5842 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Zhao S, White DG, Schroeder CM, Lu R, Yang H, McDermott PF, Ayers S, Meng J: Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl Environ Microbiol 70, 1–7 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van TT, Chin J, Chapman T, Tran LT, Coloe PJ: Safety of raw meat and shellfish in Vietnam: an analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int J Food Microbiol 124, 217–223 (2008) [DOI] [PubMed] [Google Scholar]


