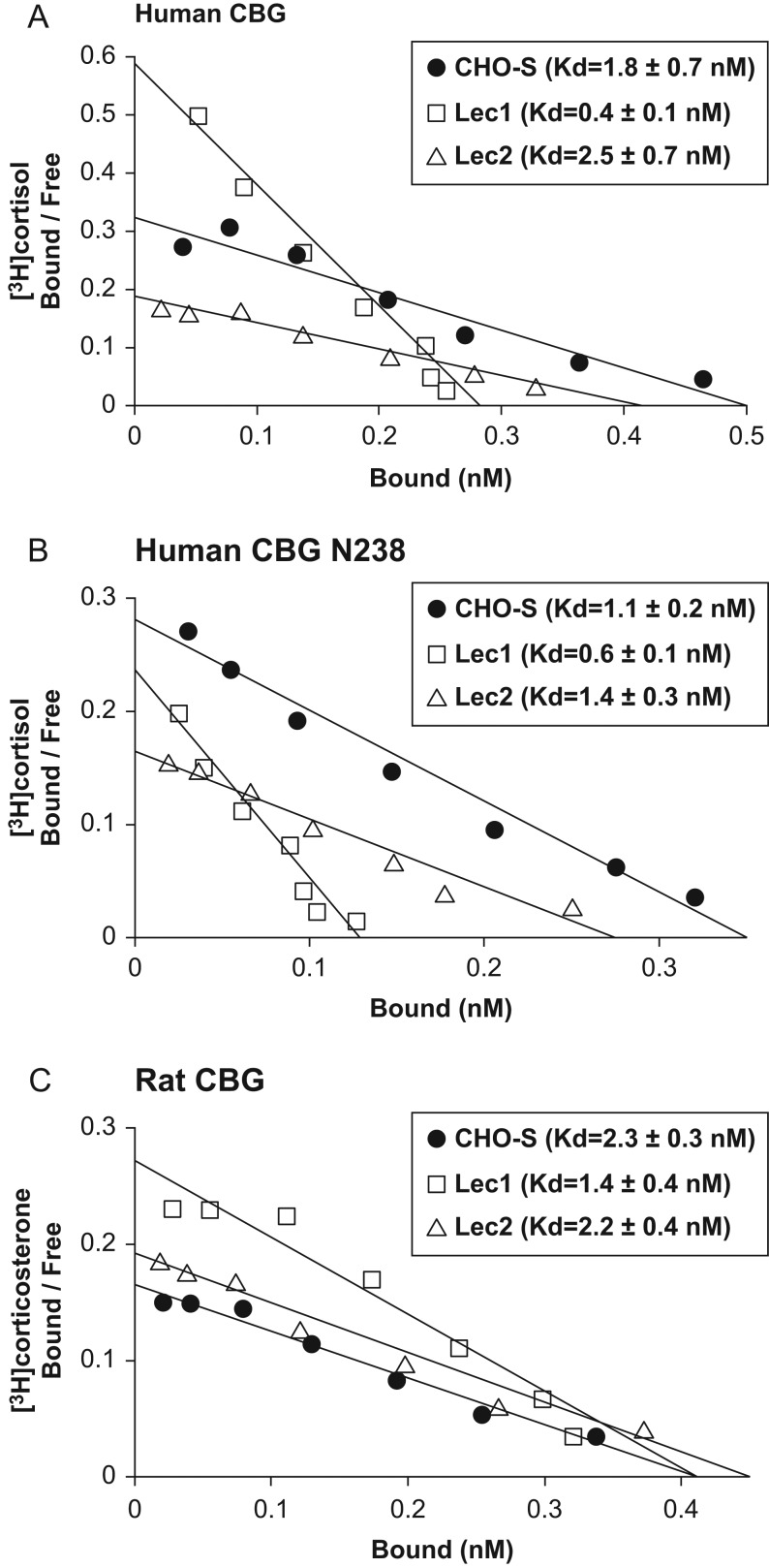
Figure 3.
Differences in N-glycosylation influence the steroid-binding affinity of human and rat CBGs. Scatchard analyses of [3H]cortisol binding to (A) human CBG and (B) a human CBG mutant with only a single N-linked glycan at N238, and (C) [3H]corticosterone binding to rat CBG. Human and rat CBGs were produced by CHO-S cells, as well as by glycosylation-deficient Lec1 or Lec2 cells, and purified by FPLC for analysis, as described (Simard et al. 2015). Representative data and linear fit are shown and mean ± s.d. dissociation constants (Kd) are shown in parentheses for replicate experiments (n = 5). One-way ANOVA followed by Tukey’s multiple comparisons test for each group revealed that the steroid-binding affinity of the CBGs produced in Lec1 cells is significantly higher than those produced in CHO-S or Lec2 cells (A and B, P < 0.01; C, P < 0.05).

 This work is licensed under a
This work is licensed under a