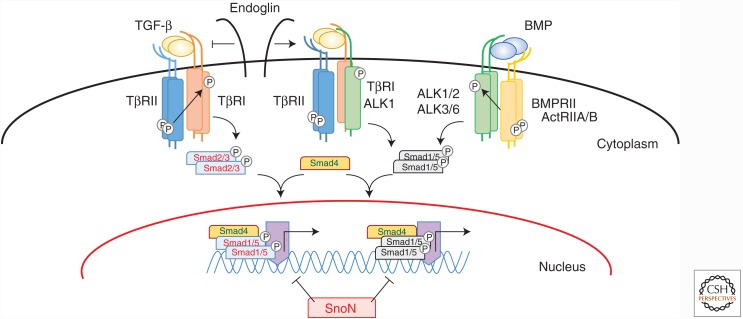
Figure 4.
TGF-β signaling pathways. TGF-βs signal by binding to a specific, heteromeric complex that includes types I and II kinase receptors (TβRI/ALK-5 and TβRII, respectively). In most cells, TGF-β binds to a complex of TβRII and TβRI/ALK-5 complex, but in endothelial cells, they can also bind to a complex of TβRII and ALK-1. The coreceptor endoglin inhibits TGF-β binding to the TβRII-ALK-5 complex and promotes TGF-β binding to the TβRII-ALK-1 complex, thus activating ALK-1 signaling. In a similar manner, bone morphogenetic proteins (BMPs) also bind to complexes of two types of receptors—that is, the type I receptors ALK-1, -2, -3, or -6, and the type II receptors BMPRII, ActRIIA, or ActRIIB. Intracellular signaling through Smad activation can be divided into two pathways. In one pathway, ALK-5 phosphorylates, and thus activates, Smad2 and Smad3, and in the other one, ALK-1, -2, -3, and -6 activate Smad1, Smad5, and Smad8. These receptor-activated Smads form heteromeric complexes with the common mediator Smad, Smad4. These complexes translocate into the nucleus, where they act as transcription factor complexes and regulate the expression of specific target genes.