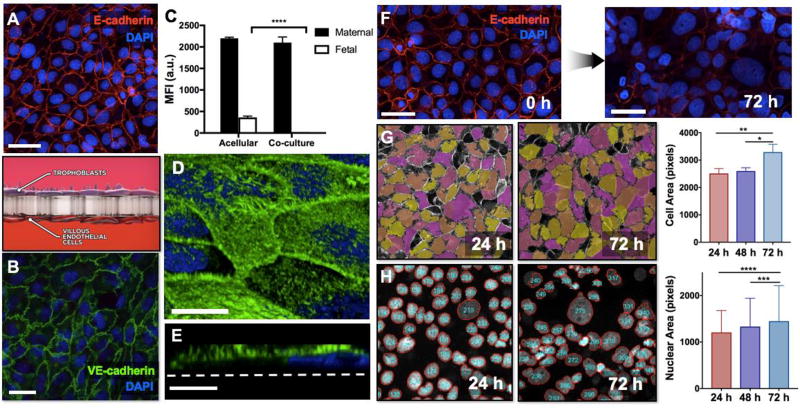
Figure 2. Microengineered in vitro placental barrier.
A. BeWo cells cultured on the upper side of the porous membrane form a continuous epithelial barrier. Red shows E-cadherin staining. Scale bar: 55 µm. B. Human placental villous endothelial cells (HPVECs) are grown to confluence on the lower side of the membrane. Widespread expression of VE-cadherin (green) illustrates the structural integrity of the endothelial tissue. Scale bar: 36 µm. C. The microengineered placental barrier effectively prevents the transfer of FITC-inulin from the maternal microchannel to the fetal compartment, demonstrating appropriate barrier function. D. Dense microvilli cover the surface of the BeWo cell population, as illustrated by F-actin staining (green). Scale bar: 20 µm. E. A cross-sectional view of actin-stained BeWo cells reveals the apical microvilli projections protruding from the cell body. Scale bar: 6 µm. F. Extended forskolin treatment induces a significant loss of intercellular junctions (red) and syncytialization-like cell fusion in BeWo cells over the course of 72 hours. Scale bars: 55 µm. Blue shows nuclear staining. G. During image analysis of E-cadherin staining, individual cells are pseudo-colored to delineate cell-cell junctions and to quantify cell area during forskolin treatment. The average area of BeWo cells increases over 72 hours, illustrating progressive cell fusion in the trophoblast population. * and ** indicate p < 0.05 and p < 0.01, respectively. H. Another method for image analysis of cell fusion is to segment DAPI-stained nuclei of BeWo cells and then measure the area of the segmented nuclei. The average size of cell nuclei increases during forskolin treatment. *** and **** indicate p < 0.001 and p < 0.0001, respectively.