Abstract
Staphylococcus aureus is a common nosocomial infection and its resistance to penicillin and methicillin antibiotics is a growing clinical problem. We previously described the development of a humanized anti-Staphylococcus enterotoxin B (SEB) antibody derived from the mouse antibody made by the 20B1 hybridoma. This antibody was humanized and characterized kinetically by Surface Plasmon Resonance demonstrating that the humanized clones retained binding to SEB. Clones were then functionally characterized in an in vitro assay demonstrating that the murine 20B1, chimeric and humanized antibodies potently inhibited SEB-induced murine splenocyte proliferation assay. Here, we describe a human cell-based screening assay, optimized by varying multiple experimental parameters that resulted in an assay that was used to demonstrate full and potent neutralization by the parental, chimeric and humanized antibodies. The replacement of Fetal Bovine Serum (FBS) with Normal Human Serum (NHS) was found to be a crucial factor in the performance of the human cell based screening assay that enabled the calculation of mAb efficacy and potency. In addition, we found that anti-SEB antibodies showed similar efficacy and potency with a triple mutant Fc region (designed to be effector function null) or a wild-type Fc region, which is in contrast to previously described studies.
Keywords: Staphylococcus aureus, SEB, humanized antibodies, in vitro
1. Introduction
S. aureus secretes a wide array of toxins that can cause symptoms ranging from food poisoning through atopic dermatitis to septic shock that can be fatal (Breuer, Wittmann et al. 2000, Drozdowski, Zhou et al. 2010, Tilahun, Rajagopalan et al. 2010). Many of these enterotoxins function as a superantigen, a bi-functional protein that cross-links MHC Class II molecules on antigen presenting cells with a significant proportion of T cell receptors, causing T cell activation. This mechanism is an effective strategy for immune evasion and increased vascular permeability (Drozdowski, Zhou et al. 2010). Staphylococcal Enterotoxin B (SEB), a 28.4kD protein, is amongst the most deleterious enterotoxins and is classified as a biological warfare agent because of its efficacy (Mantis 2005, Tsai and Pai 2009). A murine SEB-specific mAb (Murine 20B1) has been shown to modulate the host pro-inflammatory immune response in various murine models of infectious disease (Varshney, Wang et al. 2013). We undertook a humanization campaign of mAb 20B1 that generated chimeric antibodies and humanized antibodies. Humanization of mAb 20B1 was successfully performed by grafting the murine complementarity determining regions onto human variable heavy (VH) and variable light chain (VL) germ line frameworks, coupled with back mutations, producing humanized anti-SEB Abs. Binding of the test mAbs to SEB was verified by Surface Plasmon Resonance (SPR) and efficacy and potency were evaluated using a murine splenocyte SEB-induced proliferation assay (Varshney, Wang et al. 2014). We transitioned to a more clinically relevant model through the development of an SEB toxin neutralization assay that used crude freshly isolated human PBMCs. This assay was also used to determine if the lead humanized mAb should be further developed with or without the capability of human FcγR engagement.
2. Materials & methods
2.1 Generation of humanized anti-SEB antibodies
Specific details on generating humanized anti-SEB antibodies from hybridoma 20B1 have been previously described (Varshney, Wang et al. 2014). In short, a chimeric variant of the parental murine mAb 20B1 was generated by cloning the variable regions from the hybridoma and recombining with the appropriate human constant domains. The process of complementarity determining regions grafting onto human acceptor variable heavy (VH) and variable light (VL) germ line framework, designated as VH1.0 and VL1.0, respectively, combined with several specific back mutations, that were predicted to maintain binding or to stabilize antibodies, generated humanized variants of 20B1. These mAb variants contain a combination of selected mutations in VH (A49G, I69F, R71L, L78A) designated as VH 1.4 or 1.6 and combined with the VL 1.1 containing four mutations (Y36L, P44I, L46R, G66R), as described by Varshney et. al [2014]. Some mAbs were then produced with a wild type human IgG1 constant region (WT Fc) or with triple mutated human IgG1 constant region (TM Fc) which has previously been shown to eliminate binding to Fcγ receptors and associated effector function (Chappel, Isenman et al. 1991).
2.3 Development of Human Cell-Based Assay for SEB induced Proliferation
Pre-isolated leukocytes or crude PBMCs were isolated from whole blood (Bioreclamation, Westbury, NY) from anonymized middle-aged Caucasian males. Whole blood was obtained within two hours of withdraw and transferred into Becton-Dickinson vacutainer cell preparation tubes with sodium citrate, mixed and then centrifuged at 3,250 rpm for 30 min (with the brake off) at room temperature (RT). The freshly isolated PBMCs were then removed and transferred to a 50mL Falcon tube, washed with Dulbecco’s PBS, centrifuged at 1,350 rpm for 10 minutes (with the brake on) at RT. Both cell preparations were re-suspended in medium: RPMI 1640 with L-glutamine (Cellgro Manassas, VA), 10% Heat-inactivated Fetal Bovine Serum (HIFBS) (GIBCO, Life Technology, Carlsbad, CA), 1% Penicillin-Streptomycin (Cellgro, Manassas, VA) counted, and diluted to achieve a concentration of 5×106 crude PBMCs/mL. RPMI 1640 with L-glutamine media was used with 10% HIFBS [Figure 1] or with 5% heat-inactivated AB+ NHS (Invitrogen, Life Technology) [Figure 2].
Figure 1. The Humanized, Chimeric and Parental mAbs partially inhibit SEB-induced proliferation of PBMCs cultured in RPMI 1640, 10% FBS, L-glutamine, 1% Pen Strep.
PBMCs were in medium (RPMI 1640, 10% FBS, L-glutamine, 1% Pen Strep) and seeded into a 96-well plate. Test mAbs were added to the cells and then SEB was then added into the plate and incubated for 3 days. The blue circles represent an average of triplicates of the test mAb’s relative fluorescence units (RFU). The green triangles represent an average RFU of the isotype control, anti-E.tenella. The open blue circles, an average in triplicates, of the test Ab without mitogen added.
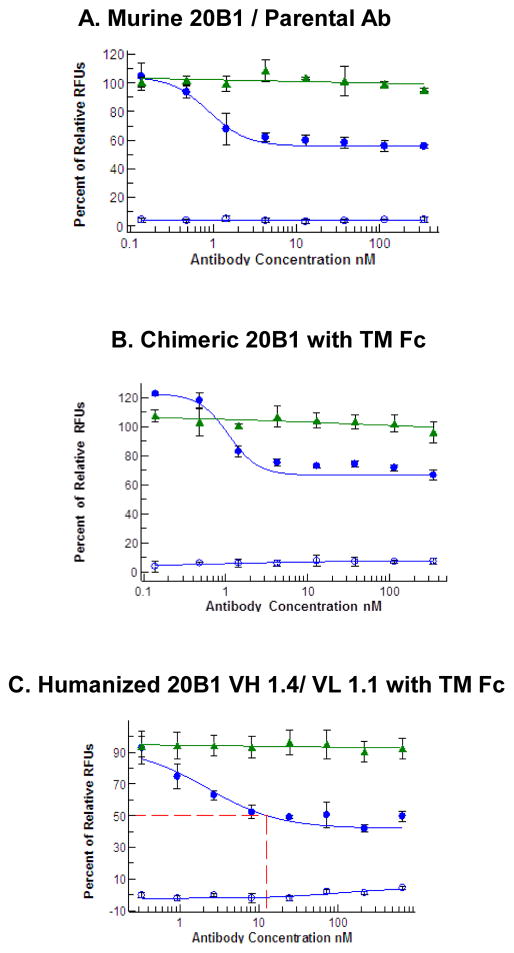
Figure 2. Replacing 10% FBS with 5% NHS reveals that antibodies completely and potently inhibit SEB-induced human PBMCs.
PBMCs were in medium (RPMI 1640, 5% Human Serum, L-glutamine, 1% Pen Strep) and were seeded into a 96-well plate. Abs were pre-mixed with 5 nM of SEB, then mixture was added into the seeded cells and incubated for 3 days. Each panel represents a different donor’s PBMCs. The green circles represent an average of triplicates of the murine 20B1 relative fluorescence units (RFU), the blue circles represent an average RFU of chimeric 20B1 and the red circles represent the average RFU of humanized 1.4/1.1. The red dotted line represents the cellular IC50.
Cells were plated in Costar 3799 96-well plates at either 1 × 105 cells/well or 5 × 104 cells/well. The optimal dynamic range was evaluated with both cell types by using a specific T-cell activating stimulus, anti-CD3ε (eBioscience, San Diego, CA), Concanavalin A (ConA) (GE healthcare Biosciences, New Jersey), or SEB. SEB was purchased from Toxin Technologies (Sarasota, FL), aliquoted to vials of 0.1 mg/mL, frozen at −80 C and used singly before appropriate disposal under local environmental health and safety guidelines. SEB was used at a maximum assay concentration of 50 ng/mL and titrated down ten-fold to a concentration of 50 pg/mL, anti-CD3ε and ConA were used at a maximum assay concentration of 100 μg/mL and titrated down ten-fold to a concentration of 10 ng/mL. Incubation times were 48, 72 and 96 hours. Sufficient humidity over each time period was maintained by placing a damp paper towel in a square lid. The experimental plate was then placed on top of the paper towel and then saran wrap™ placed over the plate. Gas exchange was facilitated by creating holes in the edges of saran wrap™.
2.4 Determination of anti SEB antibody potency
Antibodies were diluted into media each time an assay was performed and either pre-incubated with SEB or pre-mixed with cells and then serially diluted in situ in the 96-well plate. After the cells were lysed during ATP quantification, non-translucent Costar 3917 96-well plates suitable for fluorimetric analysis were used. The ViaLight Plus kit, manufactured by Lonza (Rockland, ME), with catalog # LT07-121, was used to assess total intracellular ATP as a measure of cell proliferation. To achieve optimal performance, Protocol 2 from the manufacturer’s instructions was used for each experiment. The plate was then read for 1 second on the PerkinElmer, Wallac Envision (Waltham, MA) 2104 Multi-label Reader.
2.5 Statistical Analysis
Each point, in the figures, is the average of three separate well samples. Test Abs were assayed in triplicates giving the error bars seen in Figure 1, Figure 2 and Figure 3 obtained by using excel standard error function. For the calculation of percent efficacy and Inhibitory Concentration 50% values (IC50s), the column lacking any mitogen was used as the negative control and as a baseline of cell growth (0% of SEB-induced signal). The column with no antibody and only SEB was used as the positive control and served as the readout of 100% of SEB-induced signal. The percent efficacy and IC50 were then calculated using XLfit, version 2.2.2, manufactured by ID Business Solutions (London, UK).
Figure 3. No difference demonstrated between humanized Ab with WT Fc and humanized Ab with TM Fc.
PBMCs were in medium (RPMI 1640, 5% Human Serum, L-glutamine, 1% Pen Strep) and seeded into a 96-well plate. All humanized variants, were mixed with seeded cells. Then, SEB was added and plate incubated for 3 days. The blue circles represent an average of triplicates of the test mAb’s relative fluorescence units (RFU). The red dotted line represents the cellular IC50.
The assay which used media, RPMI 1640, L-glutamine, 10% FBS, 1% Pen-strep, had three different donors, each tested twice [Figure 1]. The optimized assay, using RPMI 1640, L-glutamine, 5% NHS, 1% Pen-strep media was performed using PBMCs from five different donors and repeated at least twice in three of the five donors [Figure 2].
2.6 Ethics Statement
Blood was collected from healthy human volunteers at the Bioreclamation facility (Westbury NY), for the preparation of PBMCs or pre-fractionated leucocytes. Blood samples collected for this project were collected in accordance with Institutional Review Board approved protocols and all donors provide Informed Consent prior to blood collection.
3. Results
3.1 Development of humanized cell based assay
Murine 20B1 Ab chimeric 20B1 and all humanized variants were tested for SEB binding in solution. The humanized Abs had a 10 fold reduction in binding while the chimeric 20B1 had similar binding to murine 20B1. All humanized mAbs had comparable on-rates and minimally differing off-rates with an overall binding affinity ranging from 0.4–1.36 nM. (Varshney, Wang et al. 2014). In addition, the same antibodies exhibited full inhibition with low nM IC50 values using SEB induced proliferation of murine splenocytes (Varshney, Wang et al. 2014)
After these clones were characterized, we optimized a human cell based neutralization assay by obtaining freshly isolated PBMCs, at a concentration of 5×104 cells/100ul in RPMI 1640 with L-glutamine, 10% FBS media, were seeded in a 96-well plate that was incubated at 37 C with 5% CO2 for 3 days. 80% maximal effective concentration of SEB was established at 25 ng/mL and was either pre-mixed with mAb or added to mAb already mixed with cells. MAbs were tested in increasing concentrations up to a maximum concentration of 666 nM. Although mAbs were tested to a high concentration of 666 nM, and pre-mixed with cells, initial results (Figure 1 Panels A–C) indicated only partial inhibition, ranging from 30–55% of SEB-induced proliferation of human PBMCs.
To understand the impact of reagent addition, specifically SEB, to the in vitro assay results, we tested both pre-mixing Ab with SEB or pre-mixing Ab with the cells, following SEB treatment. Both resulted in partial inhibition (data not shown). When the parental clone was pre-mixed with SEB then added to the seeded cells, assay demonstrated 48% inhibition, while when the parental clone was pre-mixed with the cells then SEB treatment followed, the resulting inhibition was 51%. The humanized 20B1 VH 1.4/VL 1.1 resulted in 21% inhibition when pre-mixed with SEB and when pre-mixed with cells demonstrated 29% inhibition. This was within normal variation of assay variances between donors.
To further determine if the observed partial inhibition of proliferation was reflective of a true biological effect or an experimental artifact, several different controls were evaluated. One control, used in these assays, was anti-SEB mAbs incubated with cells, with no toxin added, with cell proliferation as the readout. At increasing doses of mAb no cell cytotoxicity was observed (Figure 1 Panels A–C, open blue circles). Also, an isotype-matched control Ab was used at increasing concentrations and then incubated with 25 ng/mL SEB. This was done to better understand the role of a non-specific Ab on the assay results. This control Ab did not exhibit any reduction in cell proliferation (Figure 1 Panels A–C, green triangles). The parental, chimeric and humanized mAbs were then incubated together with cells stimulated with a different stimulus, (Conconvalin A) and no reduction in cell proliferation was seen demonstrating specificity of all test mAbs for SEB (data not shown).
3.2 Full inhibition of anti-SEB humanized antibodies in optimized cell based assay
To further optimize this cell based assay system we then tested the effect of replacing FBS with commercial normal human serum at different concentrations. The use of 5% NHS eliminated artifact in cell based assay, demonstrating that parental, chimeric and humanized mAbs could fully inhibit SEB induced PBMC proliferation in different donors (Figure 2 Panels A–C). The data, in Figure 2, demonstrates that all of these Abs can fully inhibit SEB-induced human cell proliferation with low nM IC50 values and were thus consistent to the data observed of the antibodies in the murine splenocyte assay (Varshney, Wang et al. 2014). Figure 2 demonstrates the intra-assay variation observed with the parental, chimeric and humanized Abs when Abs were pre-mixed with SEB.
Using this optimized system, we tested the order in which reagents were added into 96-well plate and found that replacing serum and not the order in which reagents were added to be the contributing factor in eliminating artifact. When murine 20B1 Ab was pre-incubated with SEB, 91% inhibition was seen and when SEB was added after test Ab were incubated with cells, 104% inhibition was seen (data not shown). Humanized variant 1.4/1.1 was also tested and maintained full inhibition if it was pre-mixed with SEB or pre-mixed with cells (comparison between figure 2 and 3, respectively). This assay was then used to confirm efficacy and potency of all the humanized variants produced and enabled the selection of candidates to test in vivo.
3.3 Determination of effect from the mutations to eliminate FcγR binding
After establishing working parameters of the cell-based assay, the role of the mAb constant region was evaluated. Therefore, we tested mAbs with both triple mutant amino acid substitutions in the constant region and wild type hIgG1 Fc. We found no difference in potency between triple mutant and wild type Fc constructs. In figure 3, panels A–B, Triple mutant humanized 20B1 VH 1.4/VL 1.1 TM Fc had an IC50 of 2.3nM and 90% inhibition while humanized 20B1 VH 1.4/VL 1.1 WT Fc had an IC 50 of 1.9nM and 89% inhibition. In figure 3, panels C–D, Triple mutant humanized 20B1 VH 1.6/VL 1.1 TM Fc had an IC 50 of 0.63 nM with 93% inhibition while humanized 20B1 VH 1.6/VL 1.1 WT Fc had an IC 50 of 0.8 nM and 97% inhibition.
4. Discussion
In this study, we have demonstrated a reproducible human in vitro cell based assay whereby humanized anti-SEB antibodies could fully and potently inhibited SEB-induced human PBMC proliferation. The replacement of 10% FBS with 5% NHS demonstrated the expected full inhibitory effect of the humanized anti-SEB antibodies. In this optimized assay, the clones do not always rank repeatedly but can be used to repeatable demonstrate neutralization of SEB-specific proliferation. These assay results suggest that the use of FBS causes an experimental artifact of observed partial inhibition. This may be due in part because serum is inherently undefined and serum proteins from differing species can stimulate human cells in unexpected ways that cannot be completely defined. Cell mediated effects are unlikely because the additive serum would not contribute any cell type to the PBMCs. Further experiments to define the relative contributions of the serum percentage by a dose titration of both Normal Human and Fetal Bovine Serum, as well as any potential role for differing methods of heat inactivation of the serum would be warranted in follow up experiments.
Several human or chimeric SEB specific mAbs with varying efficacy and potency have been reported (Drozdowski, Zhou et al. 2010, Larkin, Stiles et al. 2010, Tilahun, Rajagopalan et al. 2010). Previous results by Larkin et al. suggested that SEB specific Fabs with weak potency dramatically increased potency when converted to a full length mAb despite comparable binding affinities. Subsequent SEB neutralization assays with murine L-cells demonstrated the loss of efficacy of full length mAbs. Since L-cells are devoid of Fc receptors, the authors attributed this increased potency of the mAb to the need for a functional Fc region and suggested that the Fc is important for potency. Fcγ receptor engagement could potentially be important for optimal binding and clearance of toxin but developing mAbs with a wild type Fc could affect the pharmacokinetic and pharmacodynamics properties of the mAb and result in unwanted off target effects. On the other hand, the triple mutant effector null anti-SEB antibodies will be theoretically unable to bind to Fcγ Receptors and thus trigger complement mediated cytotoxicity or antibody-dependent cellular cytotoxicity and the subsequent release of inflammatory mediators in vivo. Currently, the majority of immunomodulatory mAbs licensed by the FDA report a mutated constant region devoid of Fc Receptor engagement or low Fc-effector function (Brennan, Morton et al. 2010).
To examine this question further, we utilized our optimized in vitro assay to investigate if anti-SEB antibodies with mutations to prevent Fc Receptor binding can effectively neutralize SEB induced human PBMC proliferation as suggested by Larkin and others (Larkin, Stiles et al. 2010, Tilahun, Rajagopalan et al. 2010). Figure 3 shows, both humanized antibodies with WT Fc regions and with TM Fc fully inhibited SEB-induced proliferation with comparable IC50s. This suggests that Fc Receptor binding is not required for neutralization of SEB in an in vitro system.
The reasons for this discrepancy are not immediately obvious but may be the result of experimental differences between our respective cell based assay designs. Larkin et al. developed toxin neutralization assay that used cryopreserved cells, while we used freshly isolated PBMCs for each assay. We optimized our assay with freshly isolated cells because the dynamic range was significantly reduced when using pre-isolated leukocytes. In addition Larkin et al. added mAb after the cells were already incubated with 0.35 nM SEB and cell proliferation could occur, whereas we sequentially pre-mixed mAbs with 5 nM SEB and then added this solution to the seeded cells or added 5 nM SEB after mAb were mixed with cells. The readouts in both experiments were also different in that Larkin used IFNγ as a surrogate marker for T-cell proliferation and activation whereas we used ATP quantification as a more direct measurement of proliferation of all cells.
This data demonstrates that a fully efficacious and potent humanized antibody antagonist of SEB-induced human cell proliferation has been identified. In addition, we found that an anti-SEB antibody could be an effective neutralizing mAb, with either putative effector null Fc or wild type Fc in an in vitro assay. One or more of these mAbs has potential as a useful additional therapeutic option for the treatment of SEB+ S. aureus mediated infectious disease.
Highlights.
Fetal Bovine Serum can cause interference within a cell-based assay
Developed a human screening assay to demonstrate potency of anti-SEB antibodies.
Antibodies do not need FcγR engagement for neutralization in an in vitro system.
Acknowledgments
This work was partially supported by grant U54 AI057158 (to B. C. F.; Principal Investigator : W. Ian Lipkin, Columbia University) funded by the National Institution of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brennan FR, et al. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs. 2010;2(3):233–255. doi: 10.4161/mabs.2.3.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer K, et al. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB) Allergy. 2000;55(6):551–555. doi: 10.1034/j.1398-9995.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- Chappel MS, et al. Identification of the Fc gamma receptor class I binding site in human IgG through the use of recombinant IgG1/IgG2 hybrid and point-mutated antibodies. Proceedings of the National Academy of Sciences. 1991;88(20):9036–9040. doi: 10.1073/pnas.88.20.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdowski B, et al. Generation and characterization of high affinity human monoclonal antibodies that neutralize staphylococcal enterotoxin B. Journal of Immune Based Therapies and Vaccines. 2010;8(1):9. doi: 10.1186/1476-8518-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin EA, et al. Inhibition of toxic shock by human monoclonal antibodies against staphylococcal enterotoxin B. PloS one. 2010;5(10):e13253. doi: 10.1371/journal.pone.0013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Advanced Drug Delivery Reviews. 2005;57(9):1424–1439. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Tilahun ME, et al. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect Immun. 2010;78(6):2801–2811. doi: 10.1128/IAI.01121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WC, Pai PJ. Surface plasmon resonance-based immunosensor with oriented immobilized antibody fragments on a mixed self-assembled monolayer for the determination of staphylococcal enterotoxin B. Microchimica Acta. 2009;166(1–2):115–122. [Google Scholar]
- Varshney AK, et al. Humanized staphylococcal enterotoxin B (SEB)-specific monoclonal antibodies protect from SEB intoxication and Staphylococcus aureus infections alone or as adjunctive therapy with vancomycin. J Infect Dis. 2014;210(6):973–981. doi: 10.1093/infdis/jiu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney AK, et al. Staphylococcal Enterotoxin B-specific monoclonal antibody 20B1 successfully treats diverse Staphylococcus aureus infections. J Infect Dis. 2013;208(12):2058–2066. doi: 10.1093/infdis/jit421. [DOI] [PMC free article] [PubMed] [Google Scholar]