Abstract
How do motivation and cognitive control interact in brain and behavior? The past decade has witnessed a steady growth in studies investigating both the behavioral and the brain basis of these interactions. In this paper, I describe such interactions in the context of the dual completion model, which proposes that motivational significance influences both perceptual and executive competition. Embracing a research agenda that attempts to understand cognition-motivation interactions highlights considerable challenges faced by investigators. For example, even the standard language utilized, with terms such as “perception,” “attention,” “cognition,” and “motivation,” encourages a modular-like conceptualization of the underlying processes and mechanisms. I propose that large-scale interactions involving both task-related and valuation-related networks help understand how motivation shapes executive function. I argue that, ultimately, the mind and brain sciences need to move beyond “boxes and arrows” and fully embrace the richness and complexity of the interactions between motivation and cognition. In the last 10 years, the study in humans of the interactions of motivation with perception and cognition has grown at a fast pace. The growth has included behavioral studies characterizing the processes involved, and neuroimaging studies investigating the regions and circuits underlying the behaviors in question. This literature acknowledges the fact that perception and cognition do not happen in a vacuum but are, instead, situated in contexts that feature value. Although this assertion is uncontroversial, the mind and brain sciences have studied perception and cognition for many decades by largely extricating value from them. Fortunately, this state of affairs has now changed and the field has a newfound vigor in attempting to understand the impact of motivation on these mental functions.
The goal of the present paper is to discuss a few illustrative empirical results having in mind describing a revised version of the dual competition (Pessoa, 2009). A broader conceptual discussion then addresses the need to understand mind-brain phenomena as built from overlapping processes and mechanisms, and to transcend the limitations of language which treat mental functions as fairly independent units (“perception,” “cognition,” “action,” etc.).
Before proceeding, it should be noted that “value” and “reward” are used in this paper in an operational sense. In many experimental paradigms, participants have the opportunity to earn additional monetary compensation (“reward”) for correct and fast behavioral performance of specific trials; referred here as “potential reward.” In other manipulations, certain stimuli may be paired with extra money, and their effect on performance characterized in subsequent tasks. See Pessoa (2015) for further discussion of experimental paradigms, and Salamone et al. (2016) for discussion about value and reward.
Cognitive-motivational interactions: An example
To illustrate the types of cognition-motivation interactions considered here, let’s discuss a study in which we asked the following question (Padmala & Pessoa, 2011): Does motivation influence the selection of information? To probe this question, the effects of potential reward during a response-conflict task were investigated (Figure 1A). As we know, in response-conflict tasks, a stimulus is composed of two features that are associated with competing motor responses. As expected, behaviorally, response interference was observed and incongruent trials were slower than neutral ones (Figure 1B). Importantly, response interference was reduced during the potential-reward condition (Figure 1C). Given that response facilitation (that is, the beneficial effect of a congruent task-irrelevant item) decreased in the same condition (Figure 1C), the behavioral results supported the idea that potential reward enhanced attentional filtering, thereby reducing the influence of the task-irrelevant word item on task performance.
Figure 1. Response-conflict paradigm and behavioral data.
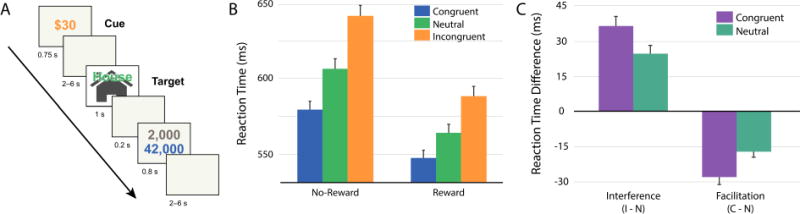
(A) Subjects performed a response conflict task (“Does the picture indicate a building or a house?”) under two contexts: during the potential reward condition (shown here), a cue stimulus (“$20”) signaled that participants would be rewarded for fast and correct performance; during the control condition (not shown here), a cue stimulus (“$00”) signaled that no reward was involved. Following a variable-length delay, a target stimulus containing a picture of a house or building was shown together with a task-irrelevant word (an incongruent condition is illustrated here). After the target stimulus, subjects were informed about the potential reward and about the total number of points accrued. (B–C) Reaction time data as a function of condition (B) or in terms of interference (incongruent vs. neutral) and facilitation (congruent vs. neutral) scores (C) illustrating the interaction patterns. C = congruent trials; I = incongruent trials; N = neutral trials.
The study above is one of a growing number of examples of how motivation (as manipulated via potential reward) exhibits selective effects on cognition. Traditional accounts describing motivation as a global activation independent of particular control demands (e.g. Duffy, 1962; Hull, 1943) have been echoed more recently by studies arguing that motivation and cognitive control can be regarded as two separate and additive — instead of interactive — factors (e.g. Kouneiher, Charron, & Koechlin, 2009). Although there is little question that motivation can have generalized, activating contributions to behavior (see Robbins & Everitt, 2007; Salamone, Correa, Farrar, Nunes, & Pardo, 2009), the findings in the past decade underscore the ability of motivation to shape behavior in specific ways, whether by reducing response conflict or task-switch costs, via selective effects on working memory, or by improving long-term memory (e.g. Aarts, van Holstein, & Cools, 2011; Botvinick & Braver, 2015; Krebs & Woldorff, 2017; Pessoa, 2013; Shohamy & Adcock, 2010). Another body of research demonstrating specific effects of motivation has investigated attentional effort (e.g. Sarter, Gehring, & Kozak, 2006).
The specificity of motivational effects is connected to the hypothesis that motivation enhances task processing so as to increase the likelihood of attaining a reward, a hypothesis consistent with a growing corpus of findings. Thus, for example, a change in the timing of subjects’ responses during a working memory task suggested a shift toward a proactive control strategy in reward contexts (Jimura, Locke, & Braver, 2010). Also, findings in response conflict tasks, such as described above, indicate that the anticipation of reward enhances stimulus processing or reduces interference from conflicting information (Harsay et al., 2011; Krebs, Boehler, & Woldorff, 2010). In these and other tasks, separate and additive effects of motivation would not have improved task performance in the same manner – that is, selectively.
Let’s return to the conflict study described above (Figure 1). In terms of brain processing, based on previous studies, it was anticipated that potential reward would enhance engagement of regions important for attention in frontal and parietal cortex and, consequently, that these regions would be better positioned to exert goal-directed control affecting visual processing. This could be accomplished by amplifying task-relevant information (Egner & Hirsch, 2005; Polk, Drake, Jonides, Smith, & Smith, 2008) and/or by improving filtering of task-irrelevant information (Polk, et al., 2008). Indeed, during the cue phase (when participants were informed whether or not a reward was possible), responses in frontal and parietal regions were stronger during the reward condition, consistent with increased attention.
During the target phase (when participants performed the actual task), we were interested in probing responses in dorsal-medial prefrontal cortex (PFC; including the anterior cingulate cortex), a region that has been suggested to be sensitive to response conflict (Botvinick, Braver, Barch, Carter, & Cohen, 2001). Although this region is engaged by many conditions (Anderson, Kinnison, & Pessoa, 2013), we assumed that, in the context of our task, the contrast of incongruent and neutral trials provided an index of the amount of response-selection demand. Similar to the behavioral data, a motivation by cognition interaction was observed, such that interference-related responses decreased during potential-reward trials. Thus, to the extent that responses in dorsal-medial PFC reflected “conflict strength,” it is reasonable to link the behavioral and neuroimaging findings: decreased behavioral interference were paralleled by decreased “conflict” responses in the brain.
We also observed the following pattern: larger cue-related responses in frontal-parietal cortex were associated with larger decreases in interference-related responses in medial PFC during the target phase. The pattern of cue and target responses was thus compatible with the notion that the up-regulation of control during the cue phase led to decreased interference during the target phase. How were cue and target responses related to the selection of visual information during the task? The relationship between cue and target responses was consistent with a mediation role for responses in visual cortex sensitive to word-related processing (in the left parahippocampal gyrus, a region that is responsive to word stimuli). During the target phase, visual responses that we interpreted to be linked to distractor processing decreased during the reward condition. Collectively, the findings suggest that participants were able to employ motivationally salient cues to upregulate attentional control mechanisms that influenced the selection of visual information in a way that reduced both behavioral conflict and conflict-related brain responses (Figure 2A).
Figure 2. Mediation relationship and coupling.
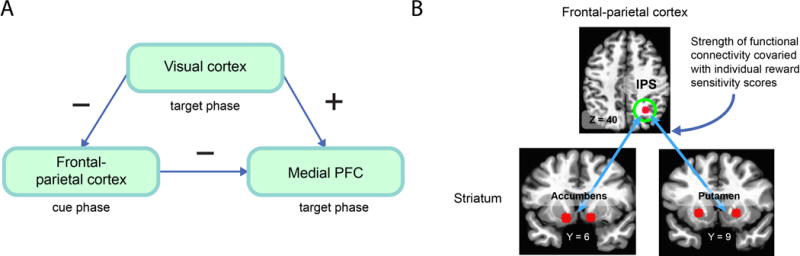
(A) The relationship between attentional control engaging fronto-parietal cortex during the cue phase and conflict-related activity in medial PFC during the subsequent target phase was hypothesized to be mediated via the amount of target/distractor processing in visual cortex. The signs indicate path relationships. For example, increased fronto-parietal responses were associated with decreased medial PFC responses. (B) Functional interactions between fronto-parietal cortex and subcortical regions involved in reward processing increased during potential reward relative to control. Across individuals, the increase in functional connection strength correlated with measures of reward sensitivity. IPS = intraparietal sulcus.
Thus far, the behavioral and brain findings were cast in terms of the task manipulation of “potential reward.” But are the findings described above a simple reflection of increased attention or are they “true” motivational effects? We will come back to this central issue and discuss it at some length later, but for now we can say that two types of relationship would strengthen the link to motivation. First, observing individual differences in brain or behavior correlated with motivation-related trait-like measures. Second, observing the involvement of brain regions thought to be important for reward-related processing. Indeed, evidence consistent with both of these factors was observed in the study, as described next.
As stated, responses to the cue stimulus were observed in frontal and parietal cortex. In addition, responses were observed in multiple subcortical regions shown in the literature to be engaged during reward-related processing (including caudate and putamen in the dorsal striatum, nucleus accumbens in the ventral striatum, as well as midbrain [e.g. see Haber & Knutson, 2010]). We reasoned that, if both frontal-parietal and subcortical regions were engaged by cues indicating potential reward, they might function as a “functional circuit;” that is, their responses covary such that they can be considered as “functionally connected” (or coupled [Friston et al., 1997; for discussion, see Pessoa, 2014]). One reason functional connectivity is important is because changes of this measure indicate changes of the relationship between regions. In particular, increases may be viewed as potential evidence for enhanced “integration.” Indeed, increased functional connectivity was detected between the frontal-parietal and subcortical regions (for instance, between the intraparietal sulcus in parietal cortex and the nucleus accumbens in ventral striatum) during the reward relative to no-reward condition (for related findings during an antisaccade task, see Harsay, et al., 2011). Notably, the strength of the coupling between cortical and subcortical areas was linearly related to individual differences in reward sensitivity (Carver & White, 1994), such that the functional interaction between these regions was stronger for participants who scored higher in this dimension (Figure 2B).
Taken together, our findings are consistent with a model in which motivationally salient cues upregulate control processes that bias the selection of visual information, thereby leading to more efficient stimulus processing during conflict conditions. This example illustrates a growing literature that is characterizing how motivation impacts both perception and cognition. This body of findings has motivated the dual competition model (Figure 3A) that proposes that affective/motivational significance influences competition at both the perceptual and the executive levels (Pessoa, 2009; 2013). Perceptual competition refers to processes that shape the ways visual items (or items from other sensory modalities) compete with one another. In essence, it speaks to how stimulus features or objects are selected so that they will affect behavior. Executive competition refers to processes that deal less with perceptual aspects of competition and more with functions such as attention and executive control, including “inhibition,” “shifting,” and “updating,” with the understanding that perceptual and executive competition are themselves interdependent (Figure 3B).
Figure 3. Dual competition model.
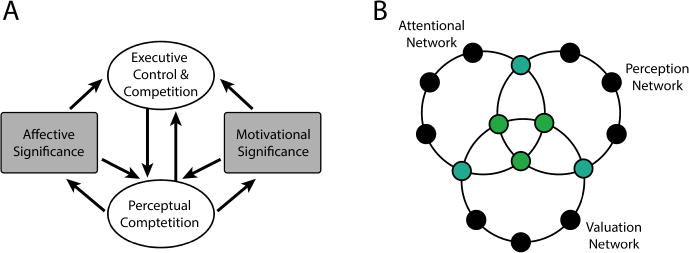
(A) Affective and motivational significance impact competition at the perceptual and executive levels. Arrows denote functional pathways that do not necessarily map to specific anatomical connections. (B) Schematic representation of some of the network interactions linked to the model.
Perceptual competition
Objects in the environment compete for limited perceptual processing capacity and control of behavior (Buschman, Siegel, Roy, & Miller, 2011; Desimone & Duncan, 1995; Pashler, 1998). Because processing capacity for vision is limited (Tsotsos, 1990), and given the constraints imposed by action selection and execution (Allport, 1987), selective attention to one part of the visual field comes at the cost of neglecting other parts. Thus, an important notion is that there is competition for resources in visual cortex (Desimone & Duncan, 1995; Grossberg, 1982).
In operationalizing perceptual competition, we can employ the concept of a priority map (Itti & Koch, 2001; Itti, Koch, & Niebur, 1998), which contains representations of spatial locations that are salient and/or behaviorally important. Although “bottom-up” factors (such as stimulus salience) and “top-down” factors (such as goal relevance) were traditionally emphasized as the major inputs to determine priority, affective/motivational variables are equally important, as now supported by a growing body of findings. By and large, that these factors are important is not surprising, as the key problem to be solved in behavior is how to select relevant information and to prepare the organism for an appropriate course of action.
For example, Anderson and colleagues (2011) reported that non-salient, task-irrelevant stimuli previously associated with reward captured attention. During an initial training phase, participants searched for a red/green target among differently colored nontargets, and received feedback at the end of each trial indicating monetary reward for correct responses. Of note, the participant’s response itself did not depend on color; rather, they were asked to discriminate the orientation of a bar within a target stimulus which was defined by its shape. In this manner, reward was associated with color, and not with a particular behavioral response. In a subsequent test phase, when no rewards were actually at stake, distractor stimuli shown in previously rewarded colors slowed visual search. Overall, the literature on both spatial and non-spatial effects of reward history on perceptual competition has flourished in the past 10 years (Anderson, 2016; Pessoa, 2015).
At an abstract level, we can conceptualize the ongoing discussion as represented in Figure 4A. In addition to stimulus-related and goal-relevant factors, we can add contributions from affective and motivational significance. To some extent, it is useful to separate the two types of significance and think of them as linked to aversive and appetitive processing, respectively. In broad terms, the architecture requires mechanisms to embed affective and motivational significance, as well as stimulus- and goal-related factors, into perceptual and selection mechanisms. Multiple implementations of the general idea may exist in the brain, such as when multiple influences converge on visual cortex, where competitive interactions help determine the items to be prioritized (Figure 4B).
Figure 4. Perception and motivational significance.
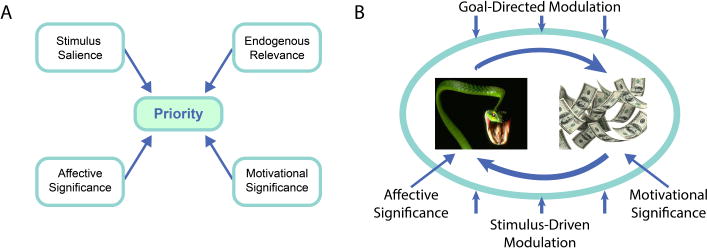
(A) Abstract representation of a priority map containing spatial locations of behavioral importance which are determined by combining multiple classes of information. (B) Perceptual competition is influenced by these factors which determine the stimulus representation to be prioritized (“wins the competition”).
Executive competition
What is executive function? Although, historically, it has been difficult to specify the composition of “the executive,” operations involved in maintaining and updating information, monitoring conflict and errors, resisting distracting information, inhibiting prepotent responses, and shifting mental sets are all important. Here we are interested in understanding how motivation influences these types of function, as exemplified in the discussion of Figure 1. Thus far, the bulk of the literature has investigated the impact of monetary reward; so, there’s great need to expand experimental manipulations to understand the broader scope of potential motivation-cognition interactions.
Sharpening and shunting executive functions
Two effects of motivation on executive function are proposed in the framework described here. First, motivation sharpens executive functions by enhancing them or by making them more efficient. In the monkey, sustained responses during the delay period of a working memory task had been shown to increase in trials involving potential reward (Watanabe, 1996). Notably, Kobayashi et al. (2002) found that such enhanced activity during the reward condition appeared to reflect an increase in the amount of transmitted information about the item being maintained in memory. Thus, reward amplified the “discriminability” of the working memory signal, leading to enhanced performance.
Second, motivation shunts (or redirects) mechanisms utilized by executive functions, increasing the likelihood of reward attainment by improving performance. For example, in the study by Jimura, Locke and Braver (2010) mentioned above, brain responses appeared to reflect a shift between control strategies, in such a way that it would benefit performance. In their working memory study, the possibility of reward did not simply increase brain responses, but it also shifted their timing. And these effects were correlated with individual differences, such that subjects with higher reward sensitivity exhibited stronger early-transient brain responses. Jimura and colleagues suggested that, in the reward condition, subjects may have adopted a more proactive control strategy to aid performance, and thus increase their chance of reward. In other words, instead of using a just-in-time reactive strategy, subjects may have, for instance, prepared a target response before the response phase itself.
Shunting between executive functions (vs. reallocating resources)
In their model of the prefrontal cortex, Miller and Cohen (2001) argued that the PFC configures processing in other parts of the brain in accordance with current task demands. Because of this general function, the PFC is critical for cognitive functions such as attention, inhibition, working memory, and other functions that support goal-directed behavior. While I believe it is unproductive to single out the PFC in isolation (it participates with other brain sectors in large-scale networks instead), it’s useful to think of executive function generally as involving the configuration of processing to support behavioral needs that are less automatic. And I propose that motivation acts to direct the way processing is configured. This is the sense in which motivation is proposed to shunt (loosely, as in switching a train from one track to another) the mechanisms utilized by executive functions. For example, in novel environments and situations, adequate levels of performance may necessitate that an individual refresh the contents of working memory, to switch the current task set, and to cancel previously planned actions – in short, updating, shifting, and/or inhibition.
Let’s discuss further the idea of shunting. The idea reframes the dual competition model in a way that eliminates casting it in terms of “resources” as previously done (Pessoa, 2009) while retaining the central notion of competition. The concept of resources has been rightly criticized for some time (e.g. Navon, 1984). While it can possibly be used in some contexts, it lacks sufficient precision to be more useful. In particular, the conceptual baggage of the term (for example, its association with the ideas of “resource theory”; e.g. Norman & Bobrow, 1975) further complicates issues. So, instead of “reallocating resources,” it is better to describe motivation as shunting the mechanisms utilized by executive functions in a way that configures processing such that behavioral requirements and motivational variables are simultaneously considered.
Informally, we can think of executive functions as linked to how specific neural circuits are engaged by task conditions. For concreteness, consider a population of neurons across multiple sites in the brain (including prefrontal and parietal cortices). Suppose that one such population is consistently engaged when updating is called for, call it PU; likewise, consider distributed populations of neurons engaged by inhibition and shifting; PI and PS, respectively (Figure 5A). If these neuronal populations P are partly shared between these three processes in space and/or time, if a given population is engaged at a particular moment in time, it will be temporarily unable to participate as effectively in other operations (Figure 5B). The extent of interference will depend on the precise mechanisms, of course, but the general idea holds, that is to say, it will depend on the overlap between, say, two neuronal populations i and j: Pi ∩ Pk.
Figure 5. Neuronal populations and shunting executive functions.
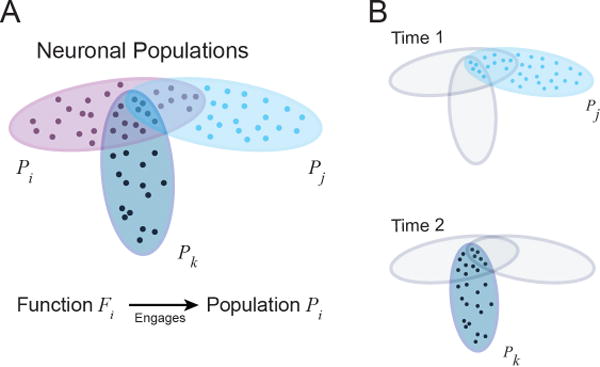
(A) Populations i, j, and k (in different colors) exhibit partial overlap. In other words, they share neuronal “real estate.” (B) Top: At any given time (Time 1), a certain population is activated in the service of carrying out an executive function. Bottom: When behavioral and motivational needs change (Time 2), processing is reconfigured such that a new population is dominant. The reconfiguration between t1 and t2 is here described as “shunting” and suggested to be an important component of motivational processing.
The advantage of the present formulation is that it eschews alluding to the vague notion of resources in favor of a physically grounded framework – one based on neural “real estate.” It also clarifies that the central idea of the framework is that of competition (as made explicit in its name).
Behavioral formulation
Although the above formulation was cast in neural terms, the ideas can be interpreted at a more abstract level more suitable for behavioral experiments. In this case, the neural populations P can be thought in terms of more abstract “processes,” which can be conveniently be labeled the same way. Furthermore, these processes are assumed to be interdependent, such that engaging one process influences the ability to engage in other ones, leading to some form of competitive interaction.
Shunting vs. sharpening
When are motivational effects due to shunting or to sharpening? One way to make some headway into distinguishing them is to investigate interference effects. Because of competition, shunting should impact not only reward-relevant operations but also other shared operations (Figure 5). A resulting prediction is that motivation can influence executive functions in a way that actually impairs behavior. Indeed, this is what we observed in a response-inhibition study (Padmala & Pessoa, 2010). We asked subjects to make a simple visual discrimination (“go” trials) unless an auditory cue was sounded (“stop” trials). Rewarding based on accurate and fast performance on “go” trials was linked to impaired inhibitory performance. That is, participants found it more difficult to withhold responding upon encountering a “stop” signal. We reasoned that, in attempting to maximize reward, they may have emphasized go-related processing to the detriment of stop-related processing. Motivation can thus be viewed as prioritizing the implementation of the rewarded task component at the expense of unrewarded components (Figure 5B).
What evidence would support the idea of sharpening executive function? In this case, the main finding should provide evidence of increased processing “efficiency,” as in the example of increased information encoded during the working memory delay (Kobayashi, et al., 2002). The study by Etzel and colleagues (2016) provides a particularly compelling illustration of sharpening (see also Boehler, Hopf, Stoppel, & Krebs, 2012). In their study, participants performed a task-switching paradigm in which trials randomly alternated between “face” (male or female) and “word” (two-syllable or not) tasks performed on composite face-plus-word stimuli. On each trial, participants were informed of the task in question by an advance cue that preceded task execution by a few seconds. Previous studies had shown that the task (in this case, face or word) can be predicted from distributed patterns of brain responses to the cue stimulus (e.g. Cole, Etzel, Zacks, Schneider, & Braver, 2011). In the study by Etzel and colleagues (2016), potential reward did not merely increase activity levels in frontal and parietal brain regions when the cue was shown but, critically, it enhanced how task-relevant information was encoded, as suggested by increased discriminability of trial type beyond that attained by the no-reward control. In general, sharpening may be related to the action of multiple neurotransmitter systems that have been shown to increase the signal-to-noise ratio of neuronal signals (including dopamine [e.g. Aarts, et al., 2011]; and acetylcholine [e.g. Sarter, Hasselmo, Bruno, & Givens, 2005]).
From boxes-and-arrows to network models of the mind-brain
A major challenge in the foregoing exposition has centered on how to use mental terms such as “perception,” “attention,” “motivation,” “cognition,” and so on. Indeed, models of the mind and brain are peppered with diagrams with boxes and arrows that link purported mental functions to each other, and/or to brain regions (or groups of regions; see Figures 2, 3A, and 4). And given that the mind-brain sciences have been doing this for more than 130 years (see Shallice, 1988), the language utilized by researchers to describe mental and neural processes is, by and large, fairly modular.
As an illustration of the conceptual issues, let’s examine an example involving reward motivation and attention. Methodologically, disentangling the contributions of cognition and motivation to brain signals is far from easy (see Hickey, Chelazzi, & Theeuwes, 2010; Peck, Jangraw, Suzuki, Efem, & Gottlieb, 2009). For example, participants may be instructed via a cue stimulus that a potential reward will result if their performance is fast and accurate. In such cases, increased brain signals may reflect enhanced attention since subjects are more likely to engage attention when a reward is at stake. Maunsell (2004, pp. 262–263) raised this important point in his discussion of monkey physiology studies:
When the effects of spatial attention are examined, subjects are motivated to direct attention to one location or another only by expectations about which location is more likely to be associated with a reward. … Such reward manipulations reliably lead to shifts in attention. … However, these experiments typically provide no basis for assigning changes preferentially to attention or to expectations about reward. In most cases, attention-related modulation could equally well be described in terms of expectation about rewards because the two are inextricably confounded.
What was at stake in his discussion was whether increased cell responses actually reflected greater attention or whether they reflected reward-related processes. As framed, the results were due to either attention or reward.
Although this example was centered on understanding potential contributions to brain signals, the problem is largely the same within the mental domain. For example, the comment above of there being “no basis for assigning changes preferentially to attention or to expectations about reward” was equally applicable to the changes in behavior observed across experiments (for further discussion of issues involving reward likelihood vs. value, see Eitam and Higgins, 2010). Indeed, the example detailed in the section “Cognitive-motivational interactions: an example” has frequently led to questions of whether the effect is one of motivation or actually an effect of attention.
In general terms, we can summarize the problem as in Figure 6. One possibility is that motivational signals modulate behavior by engaging the same set of functions that are used by cognition (such as attention), in which case, the impact on behavior can be described as “mediated by cognition” (Figure 6A). This mediation can be partial only, such that both direct (motivation-to-behavior) and indirect (motivation-via-cognition-to-behavior) effects take place. Another possibility is that cognition and affect are more intertwined, such that they jointly guide behavior (Figure 6B), in which case, although certain processes can be described as “cognitive” and others as “motivational,” the interactions between them are sufficiently high that their strict separation is more semantic than real (we will return to this scenario further below). In terms of these scenarios, the situation described by Maunsell could be portrayed by the mediation model, that is to say, mechanistically, effects of “reward” are obtained via “attentional circuits.”
Figure 6. Abstract models of the relationship between cognition and motivation.
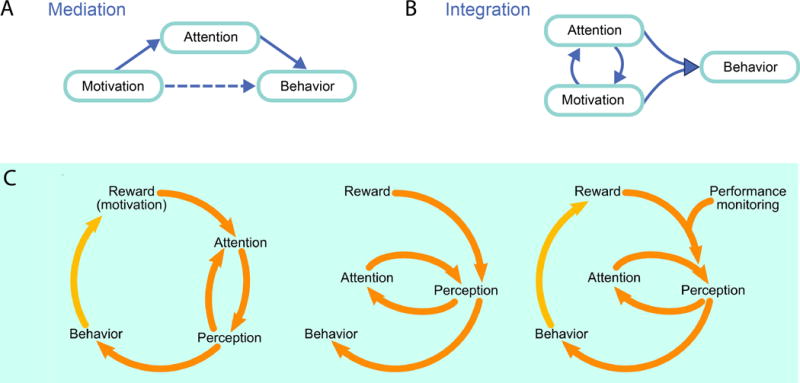
(A) In the mediation model, the influence of motivation on behavior is mediated via cognitive systems (such as attention). (B) In the integration model, cognitive and motivational systems interact sufficiently strongly that they cannot be decomposed: cognitive and motivation jointly impact behavior. (C) Additional representations of the interactions between perception and cognition with reward motivation). Part C reproduced with permission from Chelazzi et al. (1993).
More recently, Chelazzi and colleagues described multiple classes of effects of reward on visual processing that speak to the ongoing issues (Chelazzi, Perlato, Santandrea, & Della Libera, 2013; Figure 6C). Interestingly, each class was backed up by a subset of the growing literature on reward and perception/attention. It is evident that unravelling the contributions of separate mental faculties to brain and behavior is a major challenge to researchers.
Billiard-ball model of causation
The problem is not so much with diagrams such as in Figure 6A and C, but when these, implicitly perhaps, are associated with what I call the billiard-ball causal model (Figure 7A). In this model, force applied to a ball leads to its movement on the table until it hits the target ball. In this case, the reason the target ball moves is obvious; the first ball hits it, and via the force applied to the target ball, the target ball moves. But this mode of thinking, which has been very productive in the history of science, is too impoverished when complex systems – including the mind and brain – are considered.
Figure 7. Schematic diagrams of causal frameworks.
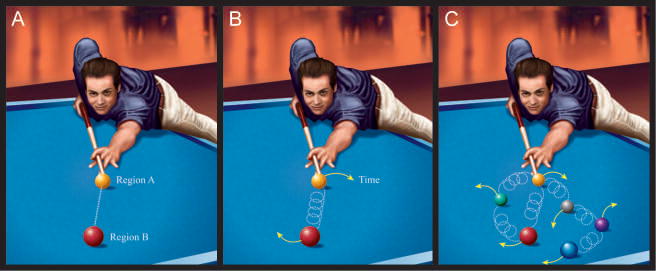
(A) Simple billiard ball scheme of causation. (B) The two balls are connected by a spring, and the goal of explanation is not to clarify where ball 2 ends up. Instead, when the initial force is applied to ball 1, the goal is to understand the evolution of the ball1—ball2 system as evolves temporally. (C) More generally, a series of springs with different coupling properties links the multiple elements in the system, which will exhibit considerably more complex dynamics.
The contention that I make is that motivational signals are not isolable from perception and cognition. This is not to say that these mental processes are so interrelated as to become one and the same thing. But when systems are not isolable, understanding the interrelatedness between “subsystems” means that we should consider interactions between systems and integration of signals as the central elements to be unraveled (see Figure 6B).
Let’s return to the billiard-ball model discussed above. Its simplicity lies in the existence of two spatially separate billiard balls that make simple contact with each other. Diagrams like those in Figure 6A place mental processes such as motivation and attention in separate boxes (like billiard balls) that can affect each other in direct, simple ways (like a ball hitting another). But this analogy will not be helpful in nondecomposable systems – like the mind and brain. Whereas thinking of causation in complex systems is much more challenging, consider the modification illustrated in Figure 7B. Here, the two balls are connected by a spring, and the goal of explanation is not to explain where ball 2 ends up. Instead, when the initial force is applied to ball 1, the goal is to understand the evolution of the ball1—ball2 system as the two balls interact with each other. More generally, a series of springs with different coupling properties links the multiple elements in the system (Figure 7C). In a related vein, an important goal for mind-brain scientists should be to understand the interactions between motivation and perception/cognition.
At the broadest level, the present discussion speaks to how we should study systems as complex as minds and brains. Dissecting phenomena in terms of their component parts seems like an unimpeachable methodology, to the extent that it can be viewed as almost an axiom of modern science (Deacon, 2011). The shift advocated here, which has been advocated by many others (e.g. Maturana & Varela, 1987; Thompson, 2007), proposes a focus not on parts but on processes, which must be understood not solely in terms of their putative constituent elements but in terms of interactions and temporal evolution.
Cognitive-motivational brain networks
What kinds of architecture support the types of interactions and intertwining between motivation and cognition alluded to above?
A first set of proposals emphasizes how “reward” regions in the ventral striatum and midbrain interface with regions important for the cognitive operations in question, as illustrated in Figure 2B. A central focus has involved interactions with the PFC, and the dorsolateral PFC in particular given its suggested role in cognitive functions. In the domain of long-term memory, interactions between the ventral striatum and hippocampus have been investigated (Shohamy & Adcock, 2010). While this this type of analysis provides information about interactions, its focus on pairs of region is overly limited.
Another approach conceptualizes the interactions in terms of networks. For instance, based on a meta-analysis of neuroimaging data, Parro, Dixon, and Christoff (2017) suggested that four networks interact during cognitive-motivational exchanges (Figure 8A): a frontoparietal control network, a sensory valuation network, an interoception/salience network, and a dopaminergic midbrain-striatal network. The scheme by Parro and colleagues thus proposes that large-scale communication is important during interactions between motivation and cognition. However, the representation displayed in Figure 8A, at least implicitly, it does not do full justice to the extensive exchanges that are observed.
Figure 8. Network models of cognition-motivation interactions.
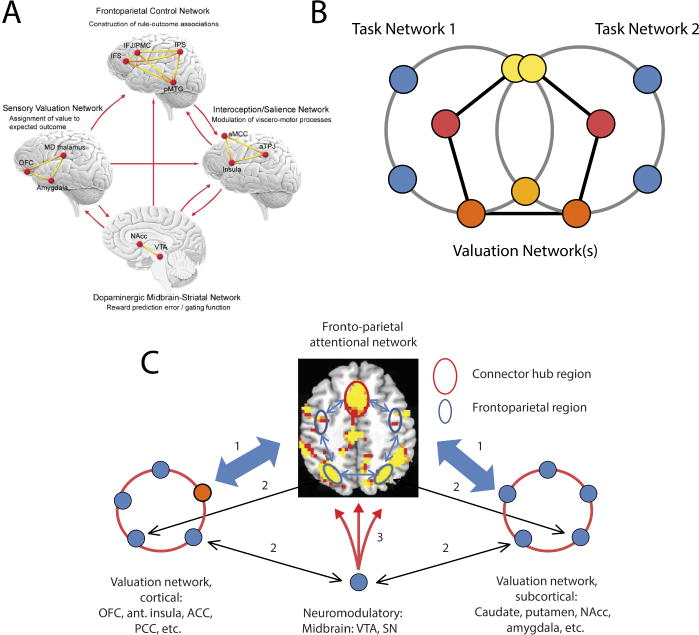
(A) Scheme proposed by Parro et al. (2017) to described the main networks involved and their interactions. (B) Abstract representation of interactions between “task” and “valuation” networks. Note that network overlap is a central feature of the organization. In particular, the overlap between the three networks at hubs regions (yellow) confers these regions with important integration and distribution properties. (C) Network representation focusing on the fronto-parietal network important for attention and multiple valuation networks. Note that the medial PFC (orange outlined) is proposed to be a hub at the interface between cognitive and valuation networks. Part A reproduced with permission from Parro et al. (2017); part C reproduced with permission from Pessoa and Engelmann (2010).
For example, in a previous study, we sought to understand network properties of brain regions during cognitive-motivational task conditions (Kinnison, Padmala, Choi, & Pessoa, 2012). Graph-theory analysis was used to characterize how the reward cue altered network organization in the task shown in Figure 1. During the control condition (no reward), clustering of the regions engaged by the task based on the similarity of responses to the cue stimulus detected two communities (or clusters of regions), one comprised of cortical regions and another with subcortical regions (plus the anterior cingulate cortex) (Figure 9A). However, when reward cues were shown, the two communities became considerably more integrated (assessed via the graph measure of efficiency). Investigation of pairwise functional connections between regions in the separate communities revealed that, with reward, changes were extensive and distributed (Figure 9B). For example, the caudate (labeled “Caud”) and the nucleus accumbens (labeled NAcc) exhibited increases in coupling to nearly all cortical regions that were systematically engaged by the reward cue (compare with the relationship illustrated in Figure 2B).
Figure 9. Cognitive-motivational integration.
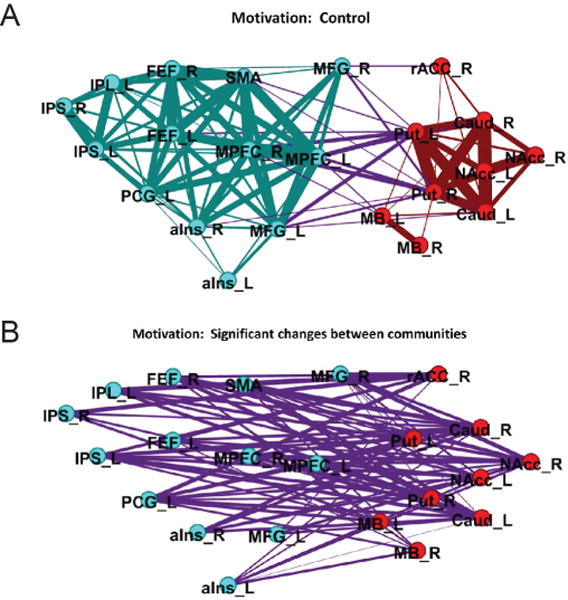
(A) Representation of the two communities (or clusters of regions) during the control condition (no reward). Nodes are colored to show community organization (red for subcortical community, teal for cortical community). Edges are also colored according to communities with between-community edges colored purple. (B) Changes in reward vs. control connectivity between the two communities (only robust [“significant”] changes are shown]. For abbreviations, see original paper. Reproduced with permission from Kinnison, Padmala, Choi, and Pessoa (2012).
Because representations such as shown in Figure 8A highlight a few core regions, they don’t fully capture the flexible and context-dependent nature of interactions. Thus, cognitive-motivational interactions can be understood in terms of the interfacing between “task” and “valuation” networks (Figure 8B). Task networks refer to task- and context-dependent coalitions of brain regions recruited by task demands, including frontal-parietal regions engaged during task switching, response inhibition, and/or working memory. Valuation networks are important for determining affective/motivational significance and involve both subcortical regions, such as the amygdala and the ventral striatum, and cortical ones, such as the orbitofrontal cortex, to name some prominent examples.
Interactions between task and valuation networks take place via multiple types of interaction involving both direct and indirect anatomical connections (Figure 8C). Critically, however, these networks are not disjoint but overlap at hub regions at the intersection of task and valuation networks (see Figure 8B). Hubs are highly connected and central regions that play a key role in information communication between different parts of a network (Guimera & Nunes Amaral, 2005; Pessoa, 2014; Sporns, 2011). In the present context, two prominent hub regions are the dorsal-medial PFC and the anterior insula.
Dorsal-medial prefrontal cortex (including the anterior cingulate cortex) plays a prominent role at the interface between diverse networks because of its participation in integrating multiple signals, including cognitive and motivational ones (e.g., Devinsky, Morrell, & Vogt, 1995; Figure 8C). This region is involved in multiple executive functions, such as conflict detection, error likelihood processing, and error monitoring (Alexander & Brown, 2011). It is also important for attentional processing more generally (Pessoa & Ungerleider, 2004). Dorsal-medial PFC is also reliably engaged during conditions involving reward manipulations (Parro, et al., 2017), as well as negative processing (Pessoa, 2013; Shackman et al., 2011).
The anterior insula is important for interoception, which involves monitoring the state not just of the viscera (Craig, 2002, 2009) but of the entire body (Paulus & Stein, 2006). Moreover, potential reward reliably engages the anterior insula (Hayes, Duncan, Xu, & Northoff, 2014; Parro, et al., 2017), which is also reliably recruited by long-term memory, working memory, task switching, attention, and many other cognitive processes (Van Snellenberg & Wager, 2010). Indeed, a recent analysis of the functional diversity of brain regions, the anterior insula emerged as one of the most diverse (Anderson, et al., 2013; see also Uddin, Kinnison, Pessoa, & Anderson, 2013).
The goal of briefly reviewing some of the functions of these two structures was to illustrate the general principle that brain networks should be conceptualized as inherently overlapping. In the present context, the importance of these regions in cognition-motivation interactions is captured by their proposed roles as hub regions that play a prominent role in the integration of information by mixing signals with distinct compositions (Figure 8B–C). Notably, these regions also distribute such intermixed signals to multiple brain regions.
A promising approach to understand overlapping networks is to allow regions to belong to multiple networks simultaneously (Gopalan & Blei, 2013). When we applied this approach to fMRI data during both taskless (“rest”) and task conditions, it detected commonly observed communities, such as the task-negative (or default) network (Najafi, Mcmenamin, & Pessoa, 2016). However, the distribution of “membership values” (the extent to which each region participated in each network; 0 and 1 in the extreme case of non-overlapping networks) indicated that regions participated in multiple networks simultaneously. Distributed participation was even more evident in a community of frontal and parietal regions important for attention and executive control, consistent with a multifunctional role for these regions. Supporting this notion, we found that “membership diversity” (the extent to which regions participated across networks) during rest scans was positively related to functional diversity (which characterizes the involvement of a region in multiple mental functions, and can be assessed by interrogating large imaging databases [Anderson, et al., 2013]; see also Robinson et al., 2012). Thus, regions that participated in more communities at rest tended to be activated by a wide variety of tasks – that is, they were functionally diverse.
Emphasizing even greater levels of interaction, in a recent proposal, I outlined how emotion and motivation can be understood based on functionally integrated systems involving distributed large-scale cortical-subcortical networks that are sensitive to bodily signals (Pessoa, 2017). Overall, the high degree of signal distribution and integration in the brain provides a nexus for the intermixing of information related to perception, cognition, emotion, motivation, and action. Importantly, the functional architecture proposed consists of multiple overlapping networks that are highly dynamic and context sensitive. Thus, how a given brain region affiliates (or clusters) with a specific network shifts as a function of task demands and brain state. Therefore, more broadly, cognitive-emotional interactions need to be understood in terms of interactions that span the entire neuroaxis, that is, all levels of the brain.
Conclusions
Perception and cognition occur in contexts where both affective and motivational significance shape them. Indeed, increasingly, researchers have sought to unravel the multiple ways in which motivation interfaces with perception and cognition, thus moving away from the previously dominant mode of focusing on their “cold” aspects. Embracing this research agenda, while necessary, also brings to the surface considerable challenges at the core of studying mind and brain.
As stated, the standard language utilized, with terms such as “perception,” “attention,” “cognition,” and “motivation,” encourages a modular-like conceptualization of how the mind-brain operates. Ultimately, this might be connected to what I called the billiard-ball model of causation (at least) implicitly adopted by researchers. Although some researchers could take issue with this characterization (and argue that they do not support such a scheme, or even that researchers in the field more generally do not), I believe an important direction for future research will be to refine and probably change the vocabulary employed in the literature. One possibility will be by adopting a stance that is less centered on parts, even with the assumption that they interact, to a framework based on processes that are inherently formulated in terms of interactions and how they evolve temporally.
The integration framework described in the present paper cannot be as easily diagrammed as the typical schemes that have permeated the mind and brain sciences for more than a century, but comes closer in spirit to complex systems of the kind displayed in Figure 7C. In the framework envisaged, motivation and cognition co-evolve and co-determine one another. Perhaps new non pencil-and-paper tools to visualize models will help investigators formulate ideas that take the mind and brain sciences beyond “boxes and arrows”!
Acknowledgments
The author acknowledges funding from the National Institute of Mental Health (MH071589) and assistance with figures and references from Christian Meyer and Anastasiia Khibovska.
References
- Aarts E, van Holstein M, Cools R. Striatal Dopamine and the Interface between Motivation and Cognition. Frontiers in Psychology. 2011;2:163. doi: 10.3389/fpsyg.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature Neuroscience. 2011;14(10):1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport DA. Selection for action: Some behavioral and neurophysiological considerations of attention and action. Perspectives on perception and action. 1987;15:395–419. [Google Scholar]
- Anderson BA. The attention habit: how reward learning shapes attentional selection. Annals of the New York Academy Sciences. 2016;1369(1):24–39. doi: 10.1111/nyas.12957. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Sciences USA. 2011;108(25):10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ML, Kinnison J, Pessoa L. Describing functional diversity of brain regions and brain networks. Neuroimage. 2013;73:50–58. doi: 10.1016/j.neuroimage.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Hopf JM, Stoppel CM, Krebs RM. Motivating inhibition – reward prospect speeds up response cancellation. Cognition. 2012;125(3):498–503. doi: 10.1016/j.cognition.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Psychology. 2015;66(1):83. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Siegel M, Roy JE, Miller EK. Neural substrates of cognitive capacity limitations. Proceedings of the National Academy of Science USA. 2011;108(27):11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Perlato A, Santandrea E, Della Libera C. Rewards teach visual selective attention. Vision Research. 2013;85:58–72. doi: 10.1016/j.visres.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Cole MW, Etzel JA, Zacks JM, Schneider W, Braver TS. Rapid transfer of abstract rules to novel contexts in human lateral prefrontal cortex. Frontiers in Human Neuroscience. 2011;5:142. doi: 10.3389/fnhum.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Deacon TW. Incomplete nature: How mind emerged from matter. WW Norton & Company; 2011. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Duffy E. Activation and behavior. New York: Wiley; 1962. [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eitam B, Higgins ET. Motivation in mental accessibility: Relevance of a representation (ROAR) as a new framework. Social and personality psychology compass. 2010;4(10):951–967. doi: 10.1111/j.1751-9004.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel JA, Cole MW, Zacks JM, Kay KN, Braver TS. Reward Motivation Enhances Task Coding in Frontoparietal Cortex. Cerebral Cortex. 2016;26(4):1647–1659. doi: 10.1093/cercor/bhu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gopalan P, Blei D. Efficient discovery of overlapping communities in massive networks. Proceedings of the National Academy of Sciences. 2013;110(36):14534–14539. doi: 10.1073/pnas.1221839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S. Processing of expected and unexpected events during conditioning and attention: a psychophysiological theory. Psychological Review. 1982;89(5):529–572. [PubMed] [Google Scholar]
- Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433(7028):895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Cohen MX, Oosterhof NN, Forstmann BU, Mars RB, Ridderinkhof KR. Functional connectivity of the striatum links motivation to action control in humans. Journal of Neuroscience. 2011;31(29):10701–10711. doi: 10.1523/jneurosci.5415-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Duncan NW, Xu J, Northoff G. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neuroscience & Biobehavioral Reviews. 2014;45:350–368. doi: 10.1016/j.neubiorev.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. Journal of Neuroscience. 2010;30(33):11096–11103. doi: 10.1523/jneurosci.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CL. Principles of Behavior: An Introduction to Behavior Theory. New York: Appleton-Century-Crofts; 1943. [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nature Reviews: Neuroscience. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C, Niebur E. A model of saliency-based visual attention for rapid scene analysis. IEEE Transactions on pattern analysis and machine intelligence. 1998;20(11):1254–1259. [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnison J, Padmala S, Choi JM, Pessoa L. Network analysis reveals increased integration during emotional and motivational processing. Journal of Neuroscience. 2012;32(24):8361–8372. doi: 10.1523/jneurosci.0821-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Lauwereyns J, Koizumi M, Sakagami M, Hikosaka O. Influence of reward expectation on visuospatial processing in macaque lateral prefrontal cortex. Journal of Neurophysiology. 2002;87(3):1488–1498. doi: 10.1152/jn.00472.2001. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience. 2009;12(7):939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Krebs R, Woldorff M. Handbook of Cognitive Control. John Wiley & Sons; 2017. Cognitive control and reward. [Google Scholar]
- Krebs RM, Boehler CN, Woldorff MG. The influence of reward associations on conflict processing in the Stroop task. Cognition. 2010;117(3):341–347. doi: 10.1016/j.cognition.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana HR, Varela FJ. The tree of knowledge: The biological roots of human understanding. New Science Library/Shambhala Publications; 1987. [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends in Cognitive Sciences. 2004;8(6):261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Najafi M, Mcmenamin BW, Pessoa L. Overlapping communities reveal rich structure in large-scale brain networks during rest and task conditions. Neuroimage. 2016;135:92–106. doi: 10.1016/j.neuroimage.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D. Resources – A theoretical soup stone? Psychological Review. 1984;91(2):216–234. [Google Scholar]
- Norman DA, Bobrow DG. On data-limited and resource-limited processes. Cognitive Psychology. 1975;7:44–64. [Google Scholar]
- Padmala S, Pessoa L. Moment-to-moment fluctuations in fMRI amplitude and interregion coupling are predictive of inhibitory performance. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(2):279–297. doi: 10.3758/CABN.10.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward Reduces Conflict by Enhancing Attentional Control and Biasing Visual Cortical Processing. Journal of Cognitive Neuroscience. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parro C, Dixon ML, Christoff K. The Neural Basis of Motivational Influences on Cognitive Control: An ALE Meta-Analysis. bioRxiv. 2017:113126. doi: 10.1002/hbm.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. The Psychology of Attention. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peck CJ, Jangraw DC, Suzuki M, Efem R, Gottlieb J. Reward modulates attention independently of action value in posterior parietal cortex. Journal of Neuroscience. 2009;29(36):11182–11191. doi: 10.1523/jneurosci.1929-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. The Cognitive-Emotional Brain: From Interactions to Integration. Cambridge: MIT Press; 2013. [Google Scholar]
- Pessoa L. Understanding brain networks and brain organization. Physics of Life Reviews. 2014;11(3):400–435. doi: 10.1016/j.plrev.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Multiple influences of reward on perception and attention. Visual cognition. 2015;23(1–2):272–290. doi: 10.1080/13506285.2014.974729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. A network model of the emotional brain. Trends in Cognitive Sciences. 2017 doi: 10.1016/j.tics.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Frontiers in Neuroscience. 2010;4 doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Top-down mechanisms for working memory and attentional processes. In: Gazzaniga MS, editor. The new cognitive neurosciences. 3rd. Cambridge, MA: MIT Press; 2004. pp. 919–930. [Google Scholar]
- Polk TA, Drake RM, Jonides JJ, Smith MR, Smith EE. Attention enhances the neural processing of relevant features and suppresses the processing of irrelevant features in humans: a functional magnetic resonance imaging study of the stroop task. Journal of Neuroscience. 2008;28(51):13786–13792. doi: 10.1523/JNEUROSCI.1026-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191(3):433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PT. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60(1):117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Frontiers in Behavioral Neuroscience. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Yohn SE, López-Cruz L, San Miguel N, Correa M. Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology. Brain. 2016;139(5):1325–1347. doi: 10.1093/brain/aww050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Research Reviews. 2006;51(2):145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews. 2005;48(1):98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. From neuropsychology to mental structure. New York: Cambridge University Press; 1988. [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends in Cognitive Sciences. 2010;14(10):464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. MIT press; 2011. [Google Scholar]
- Thompson E. Mind in life: Biology, Phenomenology, and the sciences of the mind. Cambridge, MA: Harvard University Press; 2007. [Google Scholar]
- Tsotsos JK. Analyzing vision at the complexity level. Behavioral and Brain Sciences. 1990;13:423–469. [Google Scholar]
- Uddin LQ, Kinnison J, Pessoa L, Anderson ML. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. Journal of Cognitive Neuroscience. 2013;26(1):16–27. doi: 10.1162/jocn_a_00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Wager TD. Cognitive and Motivational Functions of the Human Prefrontal Cortex. In: Christensen A-L, Goldberg E, Bougakov D, editors. Luria’s legacy in the 21st century. Oxford: Oxford University Press; 2010. pp. 30–60. [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]


