Abstract
Aims
Cardiac involvement is the main determinant of poor outcomes in sarcoidosis. Right ventricular (RV) dysfunction and left ventricular (LV) late gadolinium enhancement (LGE) have been reported to be predictive of adverse outcome in non‐ischaemic cardiomyopathies. The aim of our study was to determine whether delayed RV LGE with cardiovascular magnetic resonance would be predictive of adverse events in addition to LV LGE during the long‐term follow‐up of pulmonary sarcoidosis patients.
Methods and results
Eighty‐four consecutive biopsy‐proven pulmonary sarcoidosis patients were followed for a median of 56 months [38–74] after baseline delayed contrast‐enhanced cardiac magnetic resonance. The composite primary endpoint consisted of admission for congestive heart failure, sustained ventricular tachycardia, appropriate implantable cardioverter defibrillator therapy, pacemaker implantation for high degree atrio‐ventricular block, or cardiac death. The composite secondary endpoint included all‐cause mortality in addition to the primary endpoint. RV and LV LGE were demonstrated in respectively 12 and 27 patients. Five of 10 events included in the primary endpoint occurred in the group with RV LGE. RV LGE, LV, or biventricular LGE yielded Cox hazard ratios of 8.71 [95% confidence interval (CI) 1.90–23.81], 9.22 (95% CI 1.96–43.45), and 12.09 (95% CI 3.43–42.68) for the composite primary endpoint. In a multivariate model, the predictive value of biventricular LGE for the composite primary and secondary endpoints was strongest. Kaplan–Meier event‐free survival curves were most significant for RV LGE and biventricular LGE (log rank with P < 0.001).
Conclusions
Biventricular LGE at presentation is the strongest, independent predictor of adverse outcome during long‐term follow‐up. Asymptomatic myocardial scar <8% of LV mass carried a favourable long‐term outcome.
Keywords: Cardiovascular magnetic resonance, Late gadolinium enhancement, Sarcoidosis, Right ventricle, Risk stratification
Introduction
Sarcoidosis is a rare inflammatory condition of unknown aetiology, which results in granulomatous infiltration and focal myocardial scar in approximately a third of patients. Major cardiac morbidity or mortality has been reported in approximately 5–10% of sarcoidosis patients.1 Diagnosing cardiac sarcoidosis (CS) can be challenging, and several non‐invasive diagnostic imaging modalities have been used over the years. Recently, delayed contrast‐enhanced cardiac magnetic resonance (DECMR) and positron electron tomography (PET) showed most diagnostic and prognostic promise.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 DECMR has become the gold standard test for detecting and quantifying focal myocardial scar, a predictor of high degree atrio‐ventricular block (AVB), heart failure [congestive cardiac failure (CCF)], ventricular tachycardia (VT), and sudden cardiac death (SCD) in CS.2, 3, 4, 5, 6, 7, 8, 10, 11, 12, 13, 14, 15 (Table 1A and 1B) Systolic right ventricular (RV) impairment and multi‐focal RV late gadolinium enhancement (LGE) on cardiovascular magnetic resonance (CMR) and active RV granulomatous inflammation on PET have been associated with adverse outcomes in retrospective studies.2, 7, 11, 12, 16, 17, 18 We previously demonstrated the diagnostic accuracy of DECMR for CS and reported on RV involvement in pulmonary sarcoidosis utilizing CMR.19, 20 We currently report on the prognostic value of RV LGE in addition to left ventricular (LV) LGE in patients suffering from pulmonary sarcoidosis.
Table 1A.
Retrospective delayed contrast‐enhanced cardiac magnetic resonance studies evaluating the association between late gadolinium enhancement and adverse outcomes in cardiac sarcoidosis
| Authors | Study | Study population |
Follow‐up (months) |
Endpoints | Events | CMR findings | Predictive value for endpoints | |
|---|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | |||||||
| Shafee et al. (2012)5 | Retrospective single‐centre | 37 pts, large percentage symptomatic CS, 68% female, Japanese | 45 |
Primary: nsVT/sVT |
8 |
LV LGE + for secondary endpoint LV LGE in 70% |
31 | 100 |
|
Secondary: CD/VT/CCF |
11 | |||||||
| Crawford et al. (2014)7 | Retrospective multi‐centre |
51 pts, all CS, all LVEF > 35%, 84% female, 47% African American, 45% Caucasian, 47% steroids, 31 ICDs |
48 |
Composite: Death/VT/VF |
15 |
Any LGE + multi‐focal LGE + RV LGE + LV LGE in 63%, 14.5±12.1%, 13/32 also had RV LGE, these pts had more extensive LV LGE multi‐focal LGE correlated with VT/VF |
22 | 100 |
| 3 CD | 48 | 97 | ||||||
| 12 VT/VF | 100 | 97 | ||||||
| Ise et al. (2014)8 | Retrospective single‐centre |
43 pts, all CS‐LGE positive pts, 65% female, Japanese, all treated with steroids, 7 ICDs |
39 |
Composite: CD/VT/VF/CCF |
23 | LV LGE ≥ 21.9% of LV mass steroid treatment improved LVEF in pts with small amounts of LGE | 62 | 86 |
| 6 CD | ||||||||
| 11 VT/VF | ||||||||
| Nadel et al. (2015)10 | Retrospective single‐centre | 106 pts, systemic sarcoidosis ‐70% pulmonary, 32 CS‐CMR defined by LGE, 40% female, 58% steroids, 19 ICDs | 36.8 | Primary: | 12 |
LGE + for primary endpoint LGE + for secondary endpoints LV LGE only independent predictor of composite cardiovascular endpoint (HR 12.52) |
38 | 99 |
| SCD/VT/VF | 4 SCD | |||||||
| Secondary: | ||||||||
| •all‐cause mortality | 12 | 13 | 89 | |||||
| •SCD/appropriate ICD shock | 7 | 19 | 99 | |||||
| •SCD/VT/VF/AVB/CCF appropriate ICD shock | 37 | 97 | 92 | |||||
| Ekström et al. (2016)11 | Retrospective single‐centre | 59 pts, 50 CS pts, 48 with LV LGE, not reported on RV LGE, 64% female, Scandinavian, 35 ICDs | 26 |
Composite: CD/VT/VF/heart transplantation |
23 |
LV LGE > 22% LV LGE > 22% or VT/VF at presentation LV LGE extent and RVEF correlated with adverse outcome |
75 | 76 |
| 3 CD | 74 | 92 | ||||||
| 5 VF | ||||||||
| 14 VT | ||||||||
| Murtagh et al. (2016)12 | Retrospective single‐centre |
205 pts, extra‐cardiac sarcoidosis, LVEF > 50% 69% female, 59% African American, 60% steroids, 6 ICDs |
36 |
Composite: Death/VT |
12 8 ACD |
LGE + LGE in 20% of pts for every 1% increase in LGE burden the hazard for an event increased 8% | 32 | 99 |
| Agoston et al. (2016)13 | Retrospective single‐centre | 56 pts, all CS as defined by presence of LGE, 67% female, 52% steroids | 32 |
Composite: Death/appropriate ICD shock/nsVT /sVT/AVB/CCF |
16 1 CD 1 VT 10 CCF |
LV LGE > 18 g associated with adverse outcome | 54 | 96 |
| Yasuda et al. (2016)14 | Retrospective multi‐centre |
81pts, definite or suspected CS, 60.5% female, Japanese, 38.3% nsVT/sVT, 24.7% high degree AVB, 29.6% CCF, 52% steroids, 8 ICDS |
22.1 |
Composite: AVB/CCF/nsVT/sVT/appropriate ICD therapy/CD |
30 3 CD 12 VT 7 CCF 4 PM |
LV LGE in 94.9% of pts RV LGE in 57% of pts LV LGE 16.6±12.8% LGE ≥ 5.12 g/m2 sens 86%/spec 62% for VT/VF No events when LGE < 5.12 g/m2 and LV LGE ≤ 1 basal anteroseptal segment/RV LGE |
||
ACD, all‐cause death; AVB, atrio‐ventricular block; CCF, congestive cardiac failure; CD, cardiac death; CMR, cardiovascular magnetic resonance; CS, cardiac sarcoidosis; HR, hazard ratio; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; nsVT, non‐sustained ventricular tachycardia; PM, pacemaker; pts, patients; RV, right ventricle; SCD, sudden cardiac death; sVT, sustained ventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 1B.
Prospective delayed contrast‐enhanced cardiac magnetic resonance studies evaluating the prognostic value of late gadolinium enhancement in cardiac sarcoidosis
| Authors | Study | Study population | Follow‐up (months) | Endpoints | Events | CMR findings | Predictive value for endpoints | |
|---|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | |||||||
| Cheong et al. (2009)3 | Prospective single‐centre | 31 pts, asymptomatic, 84% pulmonary, 71% female, 39% steroids | 12 | 1 ACD | LV LGE in 26%, LV LGE 0.5–10%, 2/8 also RV LGE | |||
| Patel et al. (2009) 4 | Prospective single‐centre | 81 pts, few cardiac symptoms, 95% pulmonary, 62% female, 73% African American, 65% steroids, 6 ICDs | 21 |
Composite: ACD/appropriate ICD shock/PM implantation |
8 5 CD 6 ACD 2 ICD |
Any LGE + LV LGE in 26%, median 6.1% [2.3– 19%] , 14/21 also RV/RV septal LGE, LGE + pts had nine‐fold higher rate of adverse events |
29 | 97 |
| Greulich et al. (2013)6 | Prospective multi‐centre | 155 pts, systemic sarcoidosis, suspected CS, 40% female, 72% steroids, 13 ICDs | 31 |
Composite Primary: Death/ aborted SCD/appropriate ICD therapy Composite secondary: Primary endpoint and/or nsVT and/or sVT |
15 4 ACD 7 ICD therapy 15 + 20 6 sVT |
Any LGE + primary endpoint Any LGE + secondary endpoint LGE in 25.5%, median 4.4% [2.9–8.8], mostly RV septal LGE |
36 | 99 |
| 87 | 99 | |||||||
| Nagai et al. (2014)9 | Prospective single‐centre | 61 pts, no cardiac symptoms, JMHW (2006) negative, LVEF ≥ 50%, stable sarcoidosis, 89% pulmonary, not on immune‐suppressive agents, 66% female, Japanese | 50 |
Composite: Death/symptomatic arrhythmia/CCF |
1 PM 3 ACD |
LV LGE in 13% of pts 1 PM in LGE + group |
0 | 94 |
| Smedema et al. (2016)15 | Prospective multi‐centre | 84 pts, pulmonary sarcoidosis, 64% female, 75% Caucasian, 15% Asian, 71% steroids, 14 ICDs | 56 |
Composite Primary: AVB/appropriate ICD therapy/sVT/CCF/CD Composite secondary: Primary + ACD |
10 1 CD 6 ICD therapy 13 4 ACD |
Any LGE + LV LGE ≥ 7% LV LGE ≥ 22% RV LGE + biventricular LGE + any LGE + LV LGE in 32%, RV LGE in 14% |
29 | 96 |
| 35 | 95 | |||||||
| 57 | 92 | |||||||
| 42 | 93 | |||||||
| 56 | 93 | |||||||
| 29 | 91 | |||||||
ACD, all‐cause death; AVB, atrio‐ventricular block; CCF, admission for congestive cardiac failure; CD, cardiac death; CMR, cardiovascular magnetic resonance; CS, cardiac sarcoidosis; ICD, implantable cardioverter defibrillator; JMHW, Japanese Ministry of Health and Welfare; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; nsVT, non‐sustained ventricular tachycardia; PM, pacemaker; RV, right ventricle; SCD, sudden cardiac death; sVT, sustained ventricular tachycardia.
Methods
Patient population
Between July 2001 and August 2010, we prospectively followed 84 consecutive patients with histologically proven pulmonary sarcoidosis. These patients had been referred for cardiac evaluation, either because of cardiac symptoms (palpitations, congestive heart failure, (pre)syncope, and chest discomfort) or for routine cardiac assessment (Figure 1). Patients were excluded in case of standard contraindications to DECMR. Approval for our project was obtained from the local Institutional Review Board.
Figure 1.
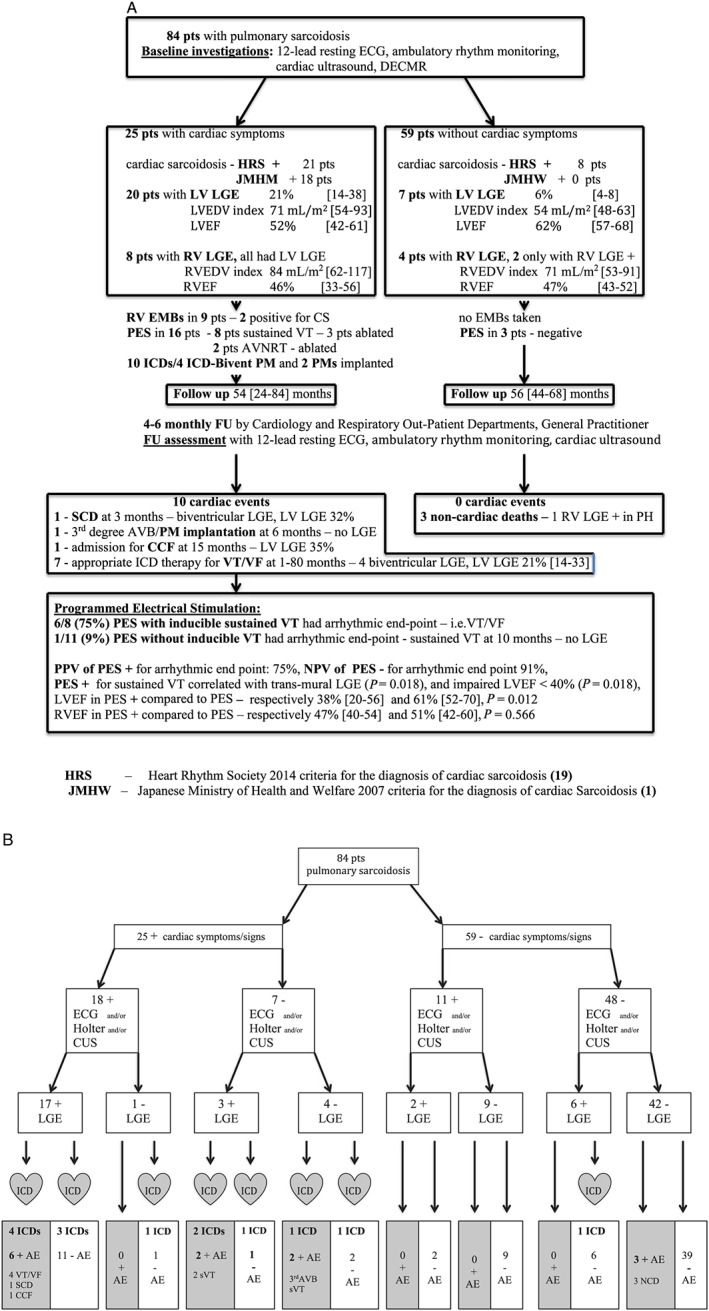
(A) Flow diagram of the baseline findings and adverse events during follow‐up included in the primary composite endpoint up in 84 sarcoidosis patients. (B) Flow diagram that demonstrates adverse outcomes as related to the findings with basic evaluation [electrocardiogram (ECG)/Holter/cardiac ultrasound (CUS)] and delayed contrast‐enhanced cardiac magnetic resonance. AVB, atrio‐ventricular block; CCF, congestive cardiac failure; DECMR, delayed contrast‐enhanced cardiac magnetic resonance; ECG,electrocardiogram; FU, follow‐up; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; PES, programmed electrical stimulation; PM, pacemaker; RV, right ventricle; RVEF, right ventricular ejection fraction; SCD, sudden cardiac death; sVT, sustained ventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Cardiovascular magnetic resonance protocol
The CMR studies were performed using a commercial 1.5 T magnetic resonance scanner with a cardiac‐dedicated, phased‐array coil. The CMR studies were electrocardiogram (ECG) triggered by standard software. Studies consisted of multi‐phase, multi‐slice, steady‐state free precession and fat‐saturated T2‐weighted spin echo (64 patients) breath‐hold sequences of the short‐axis, vertical long‐axis, and horizontal long‐axis views. The short‐axis images covered the LV and RV from base to apex. The steady‐state free precession sequences (typical repetition time: 3.5 ms; echo‐time 1.4 ms; flip‐angle 55°, temporal resolution 50 ms, voxel size 1.6 × 1.6 × 10 mm, no gap) were performed to assess regional wall‐motion abnormalities, ventricular volumes, masses, and ejection fractions [left ventricular ejection fraction (LVEF) and right ventricular ejection fraction (RVEF)]. T2‐weighted studies were performed to assess for the presence of myocardial inflammation. Contrast‐enhanced and T2‐weighted images were obtained in diastole to minimize artefact due to cardiac motion. Ten minutes after the additional administration of 0.1 mmol/kg gadolinium‐diethylenetriaminepenta‐acetic acid (Schering, Berlin, Germany), a two‐dimensional segmented inversion recovery‐gradient echo breath‐hold sequence (short axis, vertical long axis, and horizontal long axis, voxel size 1.6 × 1.6 × 10 mm, without gap), was used to assess for LGE. The inversion time (250 to 400 ms) was determined on an individual basis to obtain optimal nulling of the unenhanced myocardial signal.
Cardiovascular magnetic resonance analysis
The CMR studies were analysed offline by two experienced blinded observers who independently evaluated the study findings using commercially available software (CAAS MRV 3.4, Pie Medical Imaging, Maastricht, the Netherlands). Endocardial and epicardial contours were manually delineated in end‐diastolic and end‐systolic short‐axis slices to determine LV and RV end‐diastolic volumes (LVEDV and RVEDV), end‐systolic volume, LVEF, RVEF, and LV and RV end‐diastolic masses, which were indexed to body surface area. The presence and distribution of LGE and increased T2 signal were determined by consensus, and LV LGE was localized according to the 17‐segment model. LGE was considered present only if confirmed on both short‐axis and matching long‐axis myocardial locations. LGE was quantified by a semi‐automatic detection method using the signal intensity threshold of ≥2 SD above a remote reference region. The distribution of LGE was characterized as sub‐endocardial, mid wall, sub‐epicardial, patchy, or confluent transmural. When more than one pattern was present, the distribution was characterized on the basis of the predominant pattern. There was excellent intraobserver and interobserver correlation for ventricular volumes, masses, and ejection fractions. Interobserver agreement when determining the presence/localization of LGE was good (kappa 0.85, P < 0.001). The intra‐class correlation coefficient for LV LGE was 0.989 [0.981–0.993] (P = 0.001).
Clinical follow‐up
Scheduling of follow‐up visits was 4–6 monthly and left at the discretion of the managing clinician. Resting 12‐lead surface ECGs and 24–72 h ambulatory ECGs were performed and evaluated for intermittent intra‐ventricular or atrio‐ventricular conduction disease and/or ventricular arrhythmias. Sustained VT (sVT) was defined as VT with a rate >100 beats/min, lasting for at least 30 s. Implanted cardioverter defibrillators (ICD) or pacemakers (PM) were interrogated every 4 months and assessed for ventricular arrhythmias. At regular intervals and at the end of our study, outcome data were collected from the family physician and managing specialist.
Variables, definitions, adverse events, and composite endpoints
The composite primary endpoint consisted of newly developed AVB resulting in PM implantation, admission for CCF, sVT, appropriate ICD therapy for sVT or ventricular fibrillation (VF), or cardiac death. Appropriate ICD therapy was defined as anti‐tachycardia pacing (ATP) or shock for fast VT (R‐R <320 ms) or VF. The composite secondary endpoint included all‐cause death in addition to the primary endpoint. Peak systolic RV pressures over 40 mmHg were considered to represent pulmonary hypertension. RV end‐diastolic wall thickness over 5 mm was considered evidence of RV hypertrophy. RV dysfunction was defined as an RVEF <45% by CMR.21
Statistical analysis
All statistical analyses were performed using statistical software (Version 21.0, SPSS; Chicago, IL). Continuous normally distributed data were expressed as mean ± SD, and between‐group comparisons were made using the parametric t‐test for independent samples or Mann–Whitney U test when appropriate. In non‐normally distributed continuous data, the median and interquartile ranges were determined, and between‐group comparisons were made with the Wilcoxon test. Categorical variables were assessed using the χ2 or Fisher's exact test when appropriate. Linear regression analysis was used to determine the relationship between LGE, LVEDV, and LVEF. Univariate analyses of the risk for adverse outcome associated with selected variables were performed with the Cox proportional hazards model. A P value of <0.05 was considered statistically significant. The hazard ratio (HR) for the prediction of events was calculated for each of the outcomes using a multivariable Cox regression model; two‐tailed values of P < 0.05 were considered significant. Composite event curves were determined according to the Kaplan–Meier method, and comparisons of cumulative event rates were performed using the log‐rank test. Receiver operating characteristic (ROC) curves were used to examine the performance characteristic of %LGE mass. Area under the curve, and 95% confidence of the ROC curve, was calculated to provide a measure of the accuracy of %LGE mass to predict combined adverse outcomes.
Results
Patient characteristics
Figure 1 and Table 2 demonstrate the baseline characteristics of the included 84 patients. Twenty‐nine patients had CS according to the Heart Rhythm Society (HRS) criteria, 18 according to the Japanese Ministry of Health and Welfare criteria.22 Twenty‐five (30%) patients presented with cardiac symptoms (palpitations, (pre)syncope, chest discomfort, and congestive heart failure), while the remaining 59 patients (70%) experienced non‐specific symptoms (fatigue) or were routinely screened for CS. According to the American College of Cardiology/American Heart Association/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities, an ICD or PM were implanted in respectively 14 (four ICD/biventricular PM) and two patients after the baseline DECMR study.23 Heart failure was managed according to current optimal practice.
Table 2.
Patients' baseline characteristics
| All patients with follow‐up | 84 (100) |
| Female | 54 (64) |
| Caucasian | 63 (75) |
| Age, years | 53.3 ± 9.8 |
| Diabetes mellitus | 3 (4) |
| Hypertension | 7 (8) |
| Cardiac presentation | 25 (30) |
| Syncope | 4 (5) |
| Palpitations | 9 (11) |
| Clinical congestive heart failure | 8 (10) |
| Dyspnoea NYHA class 0 | 50 (59) |
| NYHA class 1–2 | 29 (35) |
| NYHA class 3–4 | 5 (6) |
| Sustained ventricular tachycardia | 10 (12) |
| Aborted sudden cardiac death | 1 (1) |
| Chest discomfort | 3 (4) |
| Abnormal electrocardiogram | 26 (31) |
| Pulmonary arterial hypertension | 14 (17) |
| Medication at any time | |
| Steroids | 60 (71) |
| Methotrexate | 6 (7) |
| Loop diuretics | 10 (12) |
| Spironolactone | 10 (12) |
| ACE inhibitors/ATIIRB | 11 (13) |
| Beta‐blockers | 13 (16) |
| Amiodarone | 15 (18) |
| CMR imaging parameters | |
| LVEF, % | 60 [14–84] |
| Impaired LVEF (≤50%) | 16 (19) |
| Impaired LVEF with LGE | 13 (15) |
| Impaired RVEF (≤45%) | 19 (18) |
| LVEDV, mL | 112 [88–136] |
| LVEDV index, mL/m2 | 58 [47–70] |
| LV dilation | 8 (10) |
| LV mass, g | 116 [90–142] |
| LV mass index, g/m2 | 64 [44–84] |
| LVH | 22 (26) |
| LV LGE present | 27 (32) |
| LV LGE, g | 20 [8–45] |
| LV LGE, % of LV mass | 15 [6–33] |
| RVEF, % | 47 [40–54] |
| RVEDV, mL | 140 [97–183] |
| RVEDV index, mL/m2 | 78 [58–98] |
| RV dilation | 13 (15) |
| RV mass, g | 43 [34–52] |
| RV mass index, g/m2 | 23 [18–28] |
| RVH | 11 (11) |
| RV LGE present | 12 (14) |
| T2 positive | 10/69 (14) |
| Follow up, months | 59.0 ± 22.2 [3–108] |
| Cardiac events during follow‐up | 10 (12) |
| Cardiac death | 1 (1) |
| Admission for congestive heart failure | 1 (1) |
| Appropriate ICD therapy | 7 (8) |
| Atrio‐ventricular block, pacemaker implantation | 1 (1) |
| Time to events, months | 6 (1–80) |
ACE, angiotensin‐converting enzyme; ATIIRB, angiotensine receptor blocker; CMR, cardiac magnetic resonance; EDV, end‐diastolic volume; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; NYHA, New York Heart Association; RV, right ventricle; RVEF, right ventricular ejection fraction; RVH, right ventricular hypertrophy
Values are n (%), median [IQR], or mean ± SD.
Cardiovascular magnetic resonance findings
The findings with DECMR are displayed in Table 3. Twelve patients (44%) had RV LGE, predominantly involving the right‐sided interventricular septum and/or ventricular insertion points. Two patients had isolated RV LGE. Patients with RV LGE had significantly more %LV LGE than those without RV involvement [32% (23–41) vs. 10% (2–18), P = 0.006], and RV LGE correlated with the presence of pulmonary hypertension (P = 0.022), RV hypertrophy (P = 0.050), and impaired RV function (P < 0.001).
Table 3.
Characteristics of patients with and without late gadolinium enhancement
| LGE − n = 56 | LGE + n = 28 | P value | OR (95% CI) | ||
|---|---|---|---|---|---|
| Female | 19 (34) | 11 (39) | 0.638 | ||
| Caucasian | 42 (75) | 21 (75) | 1 | ||
| Age, years | 52.4 ± 10.1 | 55.3 ± 8.9 | 0.37 | ||
| Cardiac presentation | 5 (9) | 20 (71) | <0.001 | 29.71 (8.44–104.60) | |
| Syncope | 1 (2) | 3 (11) | 0.096 | 7 (0.69–70.74) | |
| Palpitations | 2 (4) | 7 (26) | 0.004 | 9.60 (1.84–50.25) | |
| Clinical congestive heart failure | 2 (4) | 6 (21) | 0.012 | 7.86 (1.47–42.04) | |
| Sustained ventricular tachycardia | 3 (5) | 7 (25) | 0.011 | 6.30 (1.483–26.765) | |
| Aborted sudden cardiac death | 0 | 1 (4) | 0.321 | 1.13 (0.99–0.129) | |
| Chest discomfort | 0 | 3 (11) | 0.031 | ||
| Dyspnoe | NYHA 0–2 | 55 (96) | 26 (93) | 0.96 | |
| NYHA 3–4 | 2 (4) | 1 (4) | 1 | ||
| Diabetes mellitus | 3 (5) | 0 | 0.548 | ||
| Hypertension | 6 (11) | 1 (4) | 0.420 | ||
| Medication at any time | |||||
| Steroids | 36 (63) | 24 (86) | 0.019 | ||
| Methotrexate | 1 (2) | 5 (18) | 0.012 | ||
| Loop diuretics | 2 (4) | 8 (29) | 0.001 | ||
| Spironolactone | 2 (4) | 8 (29) | 0.001 | ||
| ACE inhibitors/ATIIRB | 1 (2) | 10 (36) | <0.001 | ||
| Beta–blockers | 2 (4) | 11 (39) | <0.001 | ||
| Amiodarone | 2 (4) | 12 (43) | <0.001 | ||
| Pulmonary arterial hypertension | 5 (9) | 9 (32) | 0.010 | 5.100 (1.508–17.243) | |
| CMR imaging parameters | |||||
| LVEF % | 64 [50–70] | 55 [49–72] | <0.001 | ||
| LVEDV, mL | 111 [91–131] | 132 [92–172] | 0.02 | ||
| LVEDV index, mL/m2 | 55 [48–62] | 71[49–93] | 0.007 | ||
| LV mass | 114 [94–134] | 122 [92–152] | 0.599 | ||
| LV mass index, g/m2 | 64 [44–84] | 64 [43–85] | 0.363 | ||
| LVH | 26 (46) | 15 (54) | 0.410 | ||
| LV dilation | 3 (5) | 11 (39) | <0.001 | 11.216 (2.796–44.988) | |
| LVEF ≤50% | 3 (5) | 13 (48) | <0.001 | 11.842 (2.342–59.879) | |
| RVH | 4 (7) | 7 (25) | 0.029 | 4.333 (1.147–16.366) | |
| RV mass | 39 [32–46] | 42 [35–49] | 0.753 | ||
| RV mass index, g/m2 | 22 [16–28] | 23 [20–26] | 0.964 | ||
| RV ≤45% | 6 (11) | 13 (48) | 0.011 | 7.222 (2.342–22.276) | |
| RVEF % | 48 [42–54] | 46 [36–56] | 0.288 | ||
| RVEDV, mL | 185 [143–227] | 183 [140–226] | 0.084 | ||
| RVEDV index, mL/m2 | 81 [61–101] | 96 [69–123] | 0.028 | ||
| T2 positive | 1/45 (2) | 9/24 (38) | <0.001 | 24.188 (2.834–206.451) | |
| Follow up, months | 52.4±22.1 | 61.7±21.5 | 0.62 | ||
| Events during follow–up | 2 (4) | 8 (30) | 0.001 | ||
| Time to event, months | 8 (6–10) | 4.5 (1–80) | 0.701 | ||
Values are n (%), median [IQR], or mean ± SD. Values are for all patients with sarcoidosis (84).
ACE, angiotensin‐converting enzyme; CI, confidence interval; CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVH, left ventricular hypertrophy; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OR, odds ratio; RV, right ventricle; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVH, right ventricular hypertrophy. Bold numbers are the statistically significant (P < 0.05) correlations.
The LGE involved 4–39% of LV mass, and predominantly involved the interventricular septum, and basal LV segments. LGE was equally distributed over the three myocardial layers and was patchy (56%) or confluent transmural (44%). In 16 patients (59%), three myocardial layers were involved. In 12% of patients (7/59) who were routinely evaluated, asymptomatic myocardial scar was present, though significantly smaller when compared with the symptomatic group [8% (4–20) vs. 28% (8–39), P = 0.001). LGE strongly correlated with the presence of cardiac symptoms, ECG abnormalities, VT, LVEDV, and LVEF (P < 0.001). The transmural extent of LGE correlated with segmental wall‐motion abnormalities (P < 0.001). Linear regression analysis demonstrated a decrease in LVEF of 7.27% for every 10% increase in LGE (r 2 0.392, P value < 0.001, 95% CI, −5.25% to −9.30%). For every 10% increase in LGE, the LVEDV increased 9.1 mL in volume (r 2 0.239, P < 0.001, 95% CI 5.27–12.92 mL). In patients with sVT at presentation, the median LVEF was significantly poorer, and LV mass, LVEDV index, and %LV LGE are significantly higher when compared with those without sVT at presentation [respectively 60% (53–67) vs. 51% (34–68) (P = 0.001), 55 (44–66) vs. 73 mL (50–95) (P = 0.011), 111 (80–142) vs. 145 g (95–195) (P =0.028), and 14% (range 2–38) vs. 33% (22–55) (P = 0.016)].
Follow‐up results
Figures 1A and 1B and illustrate the baseline findings and outcomes. During a median follow‐up of 56 months (1–90 months), eight patients (30%) with LGE experienced an adverse event. All adverse cardiac events occurred in patients who had presented with cardiac symptoms. A 57‐year‐old woman (New York Heart Association class 3, non‐dilated, scarred LV, LV LGE 32%, and non‐sustained VTs at rest) awaiting elective ICD implantation experienced SCD, one patient was admitted because of CCF, and six patients had appropriate ICD therapy/discharge for sVT/VF. The average annualized ICD therapy rate was 11.1%. Three layer confluent transmural LGE predicted arrhythmic events (P = 0.018) and the composite primary endpoint (P = 0.003). VT at inclusion or during follow‐up did not correlate with any specific localization grouping of LGE, such as basal septal LGE. Two patients, one presenting with palpitations and the other with pre‐syncopal symptoms, both without LGE (2/57, 4%) or T2 signal, suffered an adverse event, respectively sVT after 10 months and PM implantation for third degree AVB after 6 months. Endpoints occurred up to 80 months after baseline CMR. Three patients died of non‐cardiac conditions, respectively sepsis, respiratory failure due to extensive pulmonary fibrosis, and malignancy. None of the patients with non‐specific symptoms, such as fatigue, who had been routinely evaluated for cardiac involvement and had LGE [7/59 (12%), LV LGE 5.9 ± 3.8%] developed adverse cardiac events during follow‐up.
Predictors of events
Table 4 demonstrates the predictors of adverse events in our study. RV, LV, and biventricular LGE were the strongest predictors for adverse events. Six of eight arrhythmic endpoints occurred in patients with LVEF > 35%. Univariate Cox regression analysis found RV LGE 8.71 (95% CI 1.90–23.81), LV LGE [HR 9.22 (95% CI 1.96–43.45)], and biventricular LGE [HR 12.09 (95% CI 3.43–42.68] to be the strongest predictors of the composite primary endpoint. Multivariate Cox regression analysis, including CCF, LV LGE, biventricular LGE, any LGE, LGE with systolic ventricular dysfunction, LV dilation, and LV or RV systolic dysfunction, revealed biventricular LGE to be the best independent predictors of the composite primary and secondary endpoints at follow‐up (P < 0.001, HR 10.2, 95% CI 2.92–35.71, respectively, P = 0.001, HR 6.80, 95% CI 2.19–21.28). None of the other parameters reached statistical significance. The Kaplan–Meier event‐free survival curves found RV LGE, LV LGE [log rank (Mantel Cox) P = 0.001], and biventricular LGE [log rank (Mantel Cox) P ≤ 0.001] as the strongest predictors for the composite primary endpoint. RV LGE and biventricular LGE were the strongest predictors for the composite secondary endpoint [log rank (Mantel Cox) P ≤ 0.001] (Figure 2).
Table 4.
Univariate analysis for association with the primary composite endpoint
| No endpoints n = 74 | Primary composite endpoint n = 10 | P value | OR (95% CI) | ||
|---|---|---|---|---|---|
| Female | 48 (65) | 6 (60) | 0.76 | 0.81 (0.21–3.14) | |
| Caucasian | 56 (76) | 7 (70%) | 0.70 | 0.75 (0.175–3.21) | |
| Age, years | 53 ± 10 | 55 ± 7 | 0.66 | ||
| Cardiac presentation | 15 (20) | 10 (100) | <0.001 | 12.44 (3.037–50.994) | |
| Syncope | 2 (3) | 2 (20) | 0.02 | 9.00 (1.11–72.88) | |
| Palpitations | 4 (5) | 5 (50) | <0.001 | 17.50 (3.54–86.46) | |
| Clinical congestive heart failure | 5 (7) | 3 (30) | 0.02 | 5.91 (1.16–30.15) | |
| Sustained ventricular tachycardia | 6 (8) | 5 (50) | <0.001 | 11.33 (2.54–50.51) | |
| Aborted sudden cardiac death | 1 (1) | 0 | 0.71 | ||
| Chest discomfort | 3 (4) | 0 | 0.52 | ||
| Dyspnoea | NYHA class 0–2 | 72 (97) | 9 (90) | 0.24 | 4 (0.33‐48.66) |
| NYHA class 3–4 | 2 (3) | 1 (10) | 0.24 | ||
| Diabetes mellitus | 3 (4) | 0 | 0.52 | ||
| Hypertension | 7 (9) | 0 | 0.31 | ||
| Medication at any time | |||||
| Steroids | 51 (69) | 9 (90) | 0.17 | ||
| Methotrexate | 5 (7) | 1 (10) | 0.71 | ||
| Loop diuretics | 6 (8) | 4 (40) | 0.003 | ||
| Spironolactone | 6 (8 ) | 4 (40) | 0.003 | ||
| ACE inhibitors/ATIIRB | 6 (8) | 5 (50) | <0.001 | ||
| Beta–blockers | 5 (7) | 8 (80) | <0.001 | ||
| Amiodarone | 7 (9) | 8 (80) | <0.001 | ||
| ECG abnormalities at presentation | 19 (26) | 7 (70) | 0.004 | 6.75 (1.59–28.78) | |
| Pulmonary Hypertension | 9 (12) | 5 (50) | 0.039 | 4.24 (1.133–15.833) | |
| CMR imaging parameters | |||||
| LVEF % | 55 [48–62] | 50 [35–65] | 0.01 | ||
| LVEDV, mL | 111 [88–134] | 139 [107–171] | 0.02 | ||
| LVEDV index, mL/m2 | 58 [38–78] | 81 [64–98] | 0.02 | ||
| LVEF < 50% | 11 (15) | 5 (50) | 0.01 | 5.36 (1.33–21.68) | |
| LVEF ≤ 35% | 3 (4) | 2 (20) | 0.06 | 5.58 (0.81–38.60) | |
| LV dilation | 5 (7) | 3 (30) | 0.02 | 5.83 (1.44–29.72) | |
| LVH | 17 (23) | 5 (50) | 0.12 | ||
| LV mass | 106 [84–128] | 189 [151–227] | 0.004 | ||
| LV LGE present | 12 (4–74) | 49 [32–66] | <0.001 | 11.58 (2.26–59.39) | |
| LV LGE, % of LV | 14 [3–36] | 28 [20–36] | <0.001 | ||
| LV LGE with systolic impairment | 8 (11) | 5 (50) | 0.001 | 8.25 (1.95–34.84) | |
| RV LGE present | 6 (8) | 6 (60) | 0.002 | 9.29 (2.350–36.696) | |
| Biventricular LGE | 5 (7) | 5 (50) | 0.004 | 10.47 (2.323–47.171) | |
| RVH | 7 (9) | 4 (40) | 0.062 | 4.10 (0.989–16.691) | |
| RV mass | 42 [33–51] | 50 [38–62] | 0.305 | ||
| RV mass index, g/m2 | 22 [17–27] | 25 [19–31] | 0.1993 | ||
| RV dysfunction | 14 (19) | 5 (50) | 0.042 | 3.82 (1.101–13.285) | |
| RVEF % | 47 [41–53] | 47 [32–62] | 0.793 | ||
| RVEDV | 140 [98–182] | 137 [93–187] | 0.785 | ||
| RVEDV index, mL/m2 | 77 [57–97] | 84 [61–107] | 0.431 | ||
| T2 positive | 7/52 (13) | 3/7 (43) | 0.13 | 3.18 (0.67–15.24) | |
| Follow up, months | 53 ± 20 | 71 ± 33 | 0.01 | ||
| Time to event, months | 14.3 (1–80) | ||||
ACE, angiotensin‐converting enzyme; CI, confidence interval; CMR, cardiac magnetic resonance; ECG, electrocardiogram; LGE, late gadolinium enhancement; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; NYHA, New York Heart Association; OR, odds ratio; RV, right ventricle; RVEF,right ventricular ejection fraction; RVH, right ventricular hypertrophy. Bold numbers are the statistically significant (P < 0.05) correlations.
Values are n (%), median [IQR], or mean ± SD.
Figure 2.
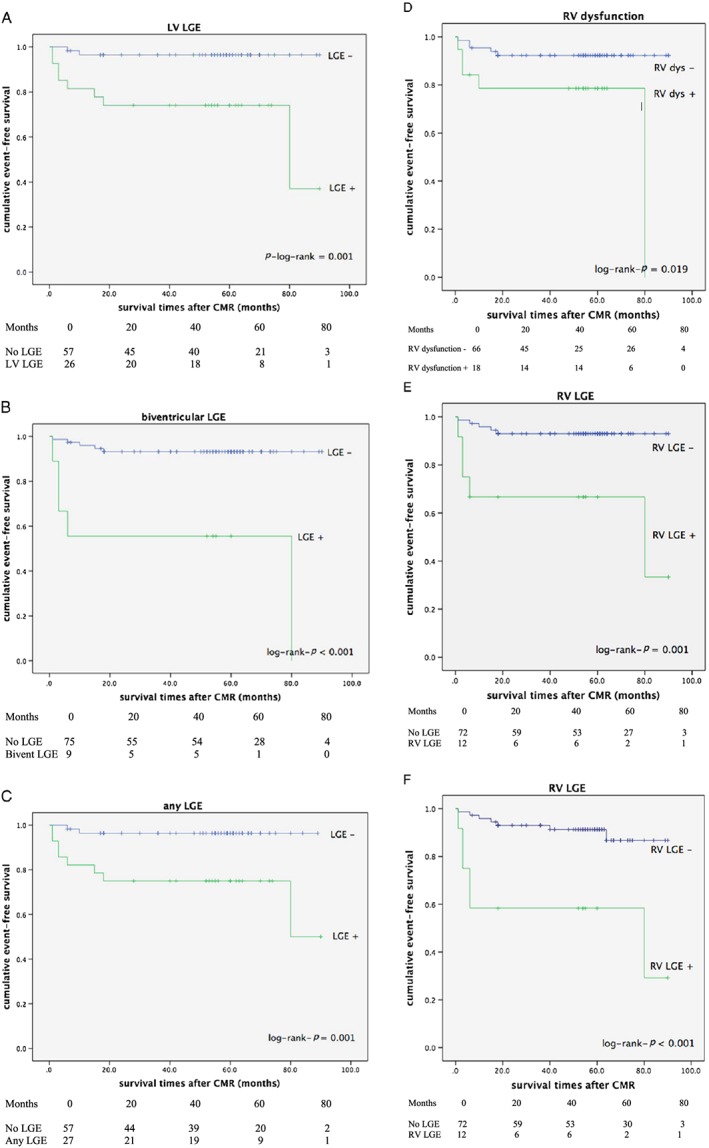
(A) Kaplan–Meier survival curve for LV LGE (composite primary endpoint). (B) Kaplan–Meier survival curve for biventricular LGE (composite primary endpoint). (C) Kaplan–Meier survival curve for any myocardial LGE (composite primary endpoint). (D) Kaplan–Meier survival curve for RV dysfunction (composite primary endpoint). (E) Kaplan–Meier survival curve for RV LGE (composite primary endpoint). (F) Kaplan–Meier survival curve for RV LGE (composite secondary endpoint). CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle; RV, right ventricle.
In the present patient population, ROC curve analysis indicated that %LV LGE had the modest ability to predict the composite primary adverse outcome (area under the curve = 0.77, 95% CI 0.58–0.95). A cut‐off level of 7% LV LGE best predicted combined adverse cardiac outcomes, with a sensitivity of 70% and a specificity of 85%. The test's positive and negative predictive values were 39% and 95%, respectively. Figures 3, 4, 5, 6 demonstrate LGE in symptomatic patients with and without adverse events during follow‐up.
Figure 3.
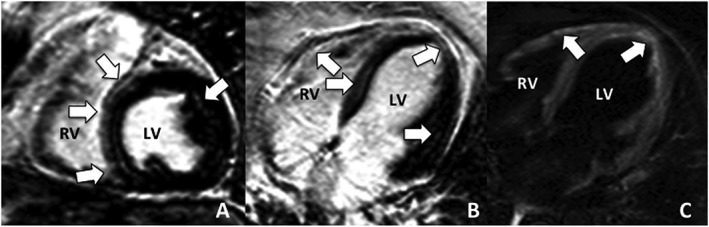
Delayed contrast‐enhanced cardiac magnetic resonance of a middle‐aged female patient who presented in CCF with frequent non‐sustained ventricular tachycardia, left bundle branch block, left ventricular ejection fraction 38% and extensive patchy late gadolinium enhancement [38% left ventricular (LV) mass], predominantly involving the right‐sided interventricular septum, right ventricular (RV) free wall, and LV mid‐epicardial and sub‐epicardial layers (arrows). A biventricular pacemaker/implantable cardioverter defibrillator was implanted. During follow‐up of 62 months, no adverse events occurred. (A) Inversion Recovery‐Gradient Echo sequence, short‐axis view; (B) Inversion Recovery‐Gradient Echo sequence, horizontal long‐axis view; (C) T2 weighted spin echo sequence–increased signal signifies inflammation of the RV free wall and LV apex.
Figure 4.
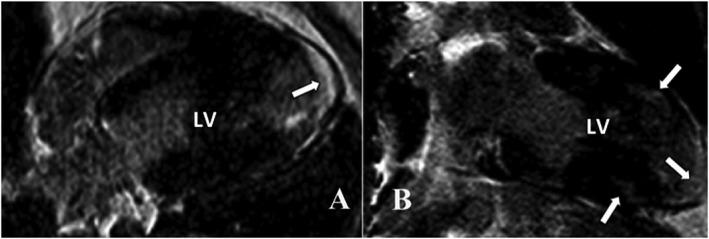
Delayed contrast‐enhanced cardiac magnetic resonance in a patient with preserved systolic left ventricular (LV) function, who presented with dyspnoea and palpitations demonstrating predominantly apical late gadolinium enhancement (arrows) (left ventricular late gadolinium enhancement 8%). Holter monitoring detected frequent episodes of non‐sustained ventricular tachycardia. 111Indium‐pentetreotide scintigraphy demonstrated active apical inflammation. An implantable cardioverter defibrillator was implanted, and immune‐suppressive and anti‐arrhythmic therapy was initiated. (A) Inversion Recovery‐Gradient Echo sequence, horizontal long‐axis view; (B) Inversion Recovery‐Gradient Echo, vertical long‐axis view.
Figure 5.
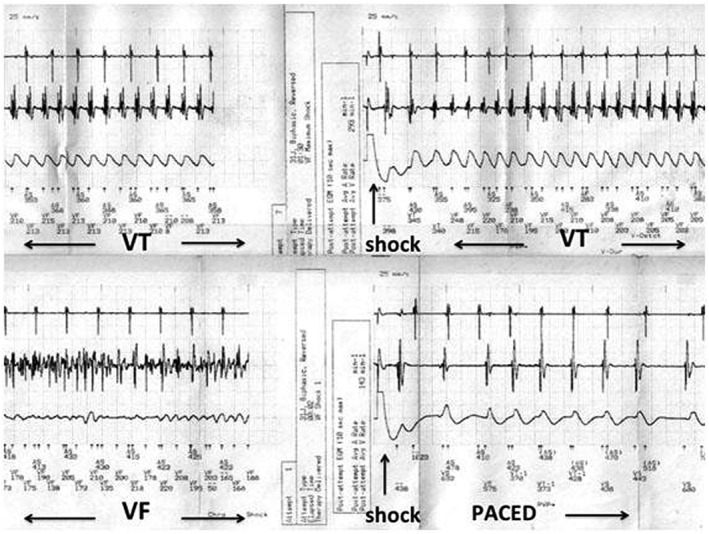
The implanted implantable cardioverter defibrillator detected episodes of sustained ventricular tachycardia after 18 months. The top strip demonstrates unsuccessful implantable cardioverter defibrillator discharge for fast monomorphic ventricular tachycardia (VT), with eventual ventricular fibrillation (VF) successfully reverted to a paced rhythm.
Figure 6.
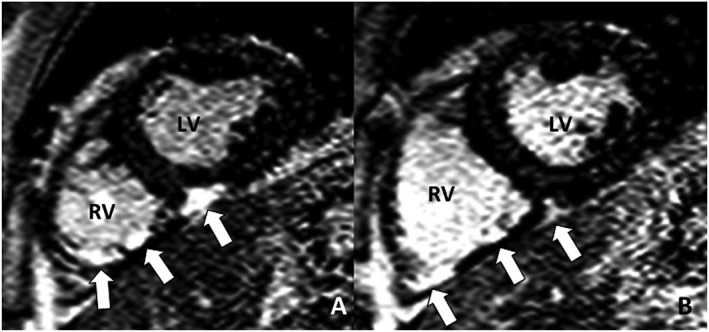
Delayed contrast‐enhanced cardiac magnetic resonance of a middle‐aged male patient who presented with pre‐syncopal symptoms because of sustained ventricular tachycardia originating from the right ventricle (RV). Delayed contrast‐enhanced cardiac magnetic resonance demonstrated extensive patchy left ventricle (LV), in addition to late gadolinium enhancement of the inferior RV segments and inferior RV insertion point (arrows). Anti‐arrhythmic and immune suppressive treatment was started, and an implantable cardioverter defibrillator was implanted. Programmed electrical stimulation on treatment was unable to elicit any monomorphic ventricular tachycardia. During follow‐up of 79 months, no adverse events occurred. (A) and (B) Inversion Recovery‐Gradient Echo sequence, short‐axis views.
One appropriate shock would be delivered for every 2.5 implanted ICDs in sarcoidosis patients, when based on the combination of cardiac symptoms, abnormal ECG and/or rhythm monitoring, and biventricular LGE or LGE > 7% of LV mass.
Discussion
Our findings demonstrate that the extent and distribution of myocardial LGE in a cohort of predominantly middle‐aged Caucasian women with chronic pulmonary sarcoidosis correlates with ventricular volumes and systolic impairment and most significantly predicts adverse (arrhythmic) outcome. Our study is the first to prospectively detail RV assessment and include T2‐weighted assessment in the majority of patients (Table 1B).
Recently, systolic RV impairment, multi‐focal RV LGE, and active RV granulomatous inflammation in CS were associated with LV LGE, sVT, and death.2, 7, 11, 12, 16, 17, 18 Our study is the first to demonstrate the direct relationship between RV LGE, RV volumes, RV systolic impairment, sVT, and death of all cause. In the absence of data on VT morphology, it remains unclear whether the prognostic relevance of RV LGE is related to more extensive biventricular arrhythmogenic substrate or whether RV LGE itself is particularly arrhythmogenic.18, 24
None of our patients with asymptomatic LGE suffered adverse cardiac events, while the majority of arrhythmic endpoints (75%) occurred in patients with LVEF > 35%. Our findings support the findings of several recent studies, which evaluated the risk of SCD in CS patients and also recorded appropriate ICD therapy in a large proportion of patients with LVEF > 35%.4, 7, 12, 22
The risk of VT and SCD in CS seems primarily related to amount and distribution of granulomas and scar and not the systolic function. Arrhythmic substrate imaging with DECMR and/or PET determines the risk of arrhythmic events more accurately than LVEF.2, 4, 7, 12, 17, 18, 22, 24 The negative predictive value of LGE negative DECMR is excellent, with only one patient with LV LGE < 8% developing an arrhythmic event after 10 months of follow‐up. In the absence of prospective data, it seems prudent to follow asymptomatic sarcoidosis patients up with ECG and cardiac ultrasound and evaluate suspected or confirmed CS with PET and/or DECMR.22 Ongoing research will determine the optimal study follow‐up interval. By reserving DECMR for sarcoidosis patients with cardiac symptoms, and/or abnormalities on basic assessment (resting ECG, ambulatory rhythm monitoring, and/or cardiac ultrasound), and implanting ICDs in patients with LGE ≥ 7%, costs could be contained and benefit optimized—in our study, one appropriate shock was delivered for every 2.5 implanted devices. Our appropriate annual ICD therapy/discharge rate of 11.1% compares with the 8.6–14.5% previously reported.22 The value of routine programmed electrical stimulation as part of risk stratification in patients without palpitations or pre‐syncopal events with LGE and LVEF > 35% remains to be determined.22
Our study supports the recommendations of the 2014 HRS guidelines concerning the use of DECMR and device implantation.22
The prognostic studies summarized in Table 1B generally report LGE in 25–30% of unselected patients cohorts and uniformly confirm the relationship between the extent of LGE and adverse outcome. Our study confirmed the favourable prognosis of small, asymptomatic myocardial scars as previously reported.7, 9, 12, 13, 14 The remarkable difference between our conclusions and those of Patel et al., who reported small asymptomatic scar in patients with mildly impaired systolic ventricular functions to be a strong risk factor for adverse events, could partly be explained by a difference in patient population and the distribution of LGE.4 Patel's cohort mainly consisted of African American women with RV LGE in 67%, a potentially high‐risk scenario. Long‐term outcomes in patients with CS have markedly improved because of modern heart failure management, including device therapy and arrhythmia ablation in selected patients.10, 11, 18, 25, 26 Current annual mortality rates range from 0–4.2/100 patients compared with 7.5–12/100 patients as previously reported by Yazaki (2001) and Fleming (1987).22 Our study is the first to include data on T2‐weighted oedema imaging. Active myocardial inflammation may increase arrhythmogenicity, but conflicting data exist concerning the efficacy of current immune‐suppressive and anti‐arrhythmic treatment in actually improving long‐term outcomes in patients with active disease.11, 12, 18, 22, 24, 25, 26 The presence of increased T2 signal in our cohort, managed with corticosteroids and methotrexate, did not correlate with adverse events during follow‐up. The accuracy for detecting active granulomatous sarcoidosis with T2‐weighted spin echo sequences is however suboptimal, and we may well have underestimated inflammatory changes.3 T2‐mapping and fluorodeoxyglucose positron emission tomography (FDG‐PET) have shown promise in diagnosing active disease and guiding immune‐suppressive management and have replaced spin echo assessment.2, 12, 18, 27
Study limitations
Our study is limited by relatively small number of, predominantly Caucasian, patients and few events. African American and Japanese populations generally have higher rates of cardiac involvement with more extensive myocardial involvement and possibly a more malignant course.4, 7, 12, 26 Intermittent ambulatory rhythm monitoring may have missed sVT. However, the long‐term outcome in our cohort still remained favourable. T1/T2 mapping would have increased the detection of interstitial fibrosis and inflammation and have potentially increased prognostic accuracy.27, 28
Conclusions
The LGE is the strongest, independent CMR predictor of future adverse cardiac events in sarcoidosis patients. RV involvement in addition to LV LGE increases risk of adverse cardiac outcomes and death of all causes. DECMR should ideally be performed in sarcoidosis patients with cardiac symptoms. Asymptomatic patients with LGE < 8% of LV mass and mildly impaired LVEF may not benefit from device therapy and be monitored. Future prospective studies will help determine the timing of DECMR studies. Current medical management including device therapy has improved survival in this condition.10, 17, 22, 26
Perspectives
Biventricular myocardial LGE is the strongest, independent CMR predictor of future adverse cardiac events in sarcoidosis patients. DECMR should ideally be performed in every sarcoidosis patient with cardiac symptoms and/or abnormal basic assessment. The presence of symptoms and extent of LGE, and not predominantly LVEF, will guide the managing clinician in when to implant an ICD. Asymptomatic Caucasian patients with limited LGE < 8% of LV mass, and preserved LVEF, may not benefit from device therapy and can be safely observed. Future research will focus on comprehensive diagnostic and prognostic strategies, which will include serological markers of disease activity and heart failure, LGE characteristics, and pre‐contrast and post‐contrast T1/T2 mapping to evaluate interstitial fibrosis and extracellular matrix volume. Hybrid PET‐CMR imaging may optimize detection and management of active inflammation. DECMR may help plan VT ablation and potentially increase success rates and outcomes.
Conflict of interest
None declared.
Acknowledgements
We are greatly indebted to Dr J. Schreurs (The Hague) who enrolled patients and provided us kindly with the follow‐up findings. The expert statistical advice of Dr P. Nelemans of the Department of Epidemiology at Maastricht University Medical Centre is greatly valued. We gratefully acknowledge Pie Medical Imaging (Maastricht, the Netherlands), which provided the CMR post‐processing software.
Smedema, J.‐P. , van Geuns, R.‐J. , Ector, J. , Heidbuchel, H. , Ainslie, G. , and Crijns, H. J. G. M. (2018) Right ventricular involvement and the extent of left ventricular enhancement with magnetic resonance predict adverse outcome in pulmonary sarcoidosis. ESC Heart Failure, 5: 157–171. doi: 10.1002/ehf2.12201.
References
- 1. Youssef G, Beanlands RSB, Birnie DH, Nery PB. Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment. Heart 2011; 97: 2078–2087. [DOI] [PubMed] [Google Scholar]
- 2. Blankstein R, Osborn M, Naya M, Waller A, Ki CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014; 63: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheong BY, Muthupillai R, Nemeth M, Lambert B, Dees D, Huber S, Castriotta R, Flamm SD. The utility of delayed‐enhancement magnetic resonance imaging for identifying non‐ischemic myocardial fibrosis in asymptomatic patients with biopsy‐proven systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26: 39–46. [PubMed] [Google Scholar]
- 4. Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009; 120: 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shafee MA, Fukuda K, Wakayama Y, Nakano M, Kondo M, Hasebe Y, Kawana A, Shimokawa H. Delayed enhancement on cardiac magnetic resonance imaging is a poor prognostic factor in patients with cardiac sarcoidosis. J Cardiol 2012; 60: 448–453. [DOI] [PubMed] [Google Scholar]
- 6. Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S, Ong P, Wagner A, Schneider S, Nassenstein KM, Sechtem U, Bruder O, Mahrhold H. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. J Am Coll Cardiol Img 2013; 6: 501–511. [DOI] [PubMed] [Google Scholar]
- 7. Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, Schuller J, Kron J, Nour KA, Cheng A, Ji SY, Feinstein S, Gupta S, Lig K, Sinno M, Abu‐Hashih S, Al‐Mallah M, Sauer WH, Ellenbogen K, Morady F, Bogun F. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014; 7: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 8. Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, Amaki M, Kanzaki H, Okamura H, Kamakura S, Shimizu W, Anzai T, Kitakaze M. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 2014; 100: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 9. Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium‐enhancement in sarcoidosis patients without cardiac manifestation. Chest 2014; 146: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 10. Nadel J, Lancefield T, Voskoboinik A, Taylor AJ. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long‐term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging 2015; 16: 1634–1641. [DOI] [PubMed] [Google Scholar]
- 11. Ekström K, Lehtonen J, Hänninen H, Kandolin R, Kivistö, Kupari M. Magnetic resonance imaging as a predictor of survival free of life‐threatening arrhythmias and transplantation in cardiac sarcoidosis. J Am Heart Assoc 2016; 5: e003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Yu Z, Addetia K, Mor‐Avi V, Moss JD, Hogarth DK, Sweiss NJ, Lang RM, Patel AR. Prognosis in myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction. Risk stratification using cardiac magnetic resonance. Circ Cardiovasc Imag 2016; 9: e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agoston‐Coldea L, Kouaho S, Sacre K. High mass (>18 g) of late gadolinium enhancement on CMR imaging is associated with major cardiac events on long‐term outcome in patients with biopsy proven extra‐cardiac sarcoidosis. Int J Cardiol 2016; 222: 950–956. [DOI] [PubMed] [Google Scholar]
- 14. Yasuda M, Iwanaga Y, Kato T, Izumi T, Inuzuka Y, Nakamura T, Miyaji Y, Kawamura T, Ikeguchi S, Inoko M, Kurita T, Miyazaki S. Risk stratification for major adverse cardiac events and ventricular tachyarrhythmia's by cardiac MRI in patients with sarcoidosis. Open Heart 2016; 3: e000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smedema JP, Snoep G, van Kroonenburgh MPG, van Geuns RJ, Dassen WR, Gorgels T, Crijns HJGM. Evaluation of the accuracy of gadolinium‐enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 16. Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, Sweiss NJ, Nguyen DT, Aleong RG, Varosy PD, Weinberger HD, Sauer WH. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol 2012; 23: 925–929. [DOI] [PubMed] [Google Scholar]
- 17. Patel AR, Klein MR, Chandra S, Spencer KT, Decara JM, Lang RM, Burke MC, Garrity ER, Hogarth DK, Archer SL, Sweiss NJ, Beshai JF. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail 2011; 13: 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muser D, Santangeli P, Patahk RK, Castro SA, Liang JJ, Magnani S, Hayashi T, Garcia FC, Hutchinson MD, Supple GE, Frankel DS, Riley MP, Lin D, Schaller RD, Desjardins B, Dixit S, Callans DJ, Zado ES, Marchlinski FE. Long‐term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electr 2016; 9: e004333. [DOI] [PubMed] [Google Scholar]
- 19. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 20. Smedema JP, van Geuns RJ, Ainslie G, Ector J, Heibuchel H, Crijns HJGM. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC Heart Failure 2017, in print. [DOI] [PMC free article] [PubMed]
- 21. Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, Raza S, Khwaja D, Brown TD, Morarji K, Liodakis E, Roughton M, Wage R, Pakrashi TC, Sharma R, Carpenter JP, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. The prevalence and prognostic significance of right ventricular systolic dysfunction in non‐ischemic dilated cardiomyopathy. Circulation 2013; 128: 1623–1636. [DOI] [PubMed] [Google Scholar]
- 22. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS Expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014; 11: 1305–1323. [DOI] [PubMed] [Google Scholar]
- 23. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeny MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities. Circulation 2008; 117: e350–e408. [DOI] [PubMed] [Google Scholar]
- 24. Jefic D, Joel B, Good E, Morady F, Rosman H, Knight B, Bogun F. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multi‐centre registry. Heart Rhythm 2009; 6: 189–195. [DOI] [PubMed] [Google Scholar]
- 25. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Kaikkonen K, Hataaja P, Kerola T, Kupari M. Cardiac sarcoidosis. Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015; 131: 624–1632. [DOI] [PubMed] [Google Scholar]
- 26. Zhou Y, Lower EE, Li H, Farhey Y, Baughman RP. Cardiac sarcoidosis: the impact of age and implanted devices on survival. Chest 2017; 151: 139–148. [DOI] [PubMed] [Google Scholar]
- 27. Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Resp Crit Care Med 2014; 189: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greulich S, Kitterer D, Latus J, Aguor E, Steubing H, Kaesemann P, Patrascu A, Greiser A, Groeninger S, Mayr A, Braun N, Alscher MD, Sechtem U, Mahrhold H. Comprehensive cardiovascular magnetic resonance assessment in patients with sarcoidosis and preserved left ventricular ejection fraction. Circ Cardiovasc Imaging 2016; 9: e005022. [DOI] [PubMed] [Google Scholar]


