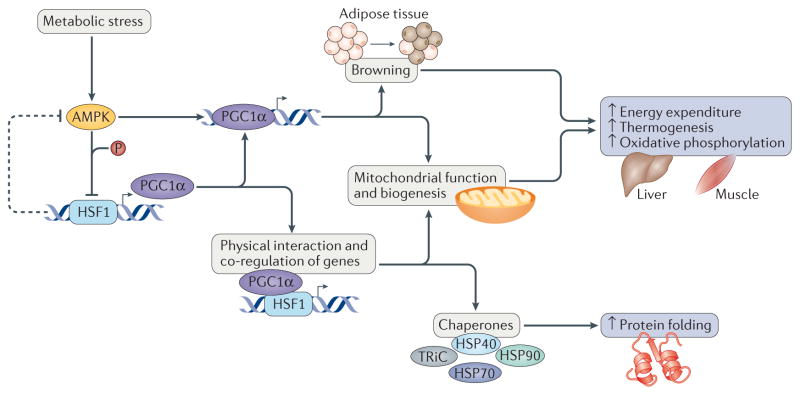
Figure 3. Heat shock transcription factor 1 at the forefront of metabolic regulation.
Heat shock transcription factor 1 (HSF1) acts as a core component of metabolic regulation through its ability to respond to metabolic and environmental stresses in key organs such as the liver and skeletal muscle. In this regard, HSF1 directly activates expression of the transcription factor peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α). Moreover, through direct protein protein interactions, PGC1α cooperates with HSF1 to activate the expression of chaperones and proteins that function in mitochondrial metabolism and biogenesis to prevent oxidative damage and increase oxidative phosphorylation48,51,53,55. The HSF1 and PGC1α network also functions in white adipose tissue browning, conferring heat production and beneficial effects on adiposity, insulin resistance and dyslipidaemia54. Intriguingly, this intricate network is simultaneously inhibited and activated by the metabolic stress sensor 5′-AMP-activated protein kinase (AMPK). AMPK phosphorylates and represses HSF1 function during nutrient deprivation, causing a proteotoxic stress response41. However, AMPK also activates PGC1α expression and transcriptional activity in adipose tissue124. This regulation focuses on modulating energy expenditure to improve metabolic fitness and stress protection. HSP40, heat shock protein 40; TRiC, TCP1 ring complex.