Abstract
Epithelial ovarian cancer (EOC) metastasizes intra-abdominally with often numerous, superficial, small-sized lesions. This so-called peritoneal carcinomatosis is difficult to treat, and peritoneal recurrences are frequently observed, leading to a poor prognosis. Underlying mechanisms of interactions between EOC and peritoneal cells are incompletely understood. This review summarizes and discusses the development of peritoneal carcinomatosis from a cell-biological perspective, focusing on characteristics of EOC and peritoneal cells. We aim to provide insight into how peritoneum facilitates tumor adhesion but limits size of lesions and depth of invasion. The development of peritoneal carcinomatosis is a multistep process that requires adaptations of EOC and peritoneal cells. Mechanisms that enable tumor adhesion and growth involve cadherin restructuring on EOC cells, integrin-mediated adhesion, and mesothelial evasion by mechanical forces driven by integrin-ligand interactions. Clinical trials targeting these mechanisms, however, showed only limited effects. Other factors that inhibit tumor growth and deep invasion are virtually unknown. Future studies are needed to elucidate the exact mechanisms that underlie the development and limited growth of peritoneal carcinomatosis. This review on development of peritoneal carcinomatosis of EOC summarizes the current knowledge and its limitations. Clarification of the stepwise process may inspire future research to investigate new treatment approaches of peritoneal carcinomatosis.
Keywords: epithelial ovarian carcinoma, pathogenesis, peritoneal barrier, peritoneal metastases, peritoneal metastasis, peritoneum
Introduction
Epithelial ovarian cancer (EOC) is the sixth most common cancer affecting women and the most common cause of gynecologic cancer-associated death. Due to absence of symptoms of EOC in early stages, approximately 80% of patients present with advanced disease. In advanced stages, widespread intra-abdominal disease with peritoneal metastases is often present, a condition also referred to as peritoneal carcinomatosis. Extra-abdominal disease occurs mainly in late stages of the disease. Patients with advanced EOC are treated with cytoreductive surgery in combination with platinum- and taxane-based chemotherapy. The aim of the surgical procedure is to achieve complete cytoreduction without macroscopically visible residual lesions, as these patients have a more favorable prognosis than patients with residual lesions after surgery, even when these lesions are less than 1 cm in diameter.1 However, cytoreductive surgery is hampered by the presence of peritoneal carcinomatosis, which is often too extensive to remove completely, especially when present on the small intestine. Although chemotherapy is generally very effective with high response rates (80%), the chance of recurrent disease in advanced-stage EOC is approximately 75%.2,3 In relapsed patients, peritoneal recurrences are frequently found, causing ominous symptoms such as ascites and bowel ileus, due to extrinsic occlusion of the bowel, or infiltration of mesentery, bowel muscles, or nerves, ultimately leading to death. In these situations, it is rare to achieve complete cure, and overall prognosis is poor.
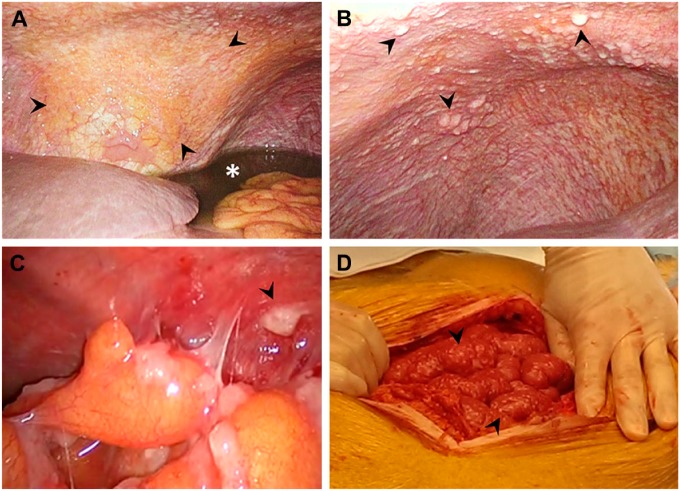
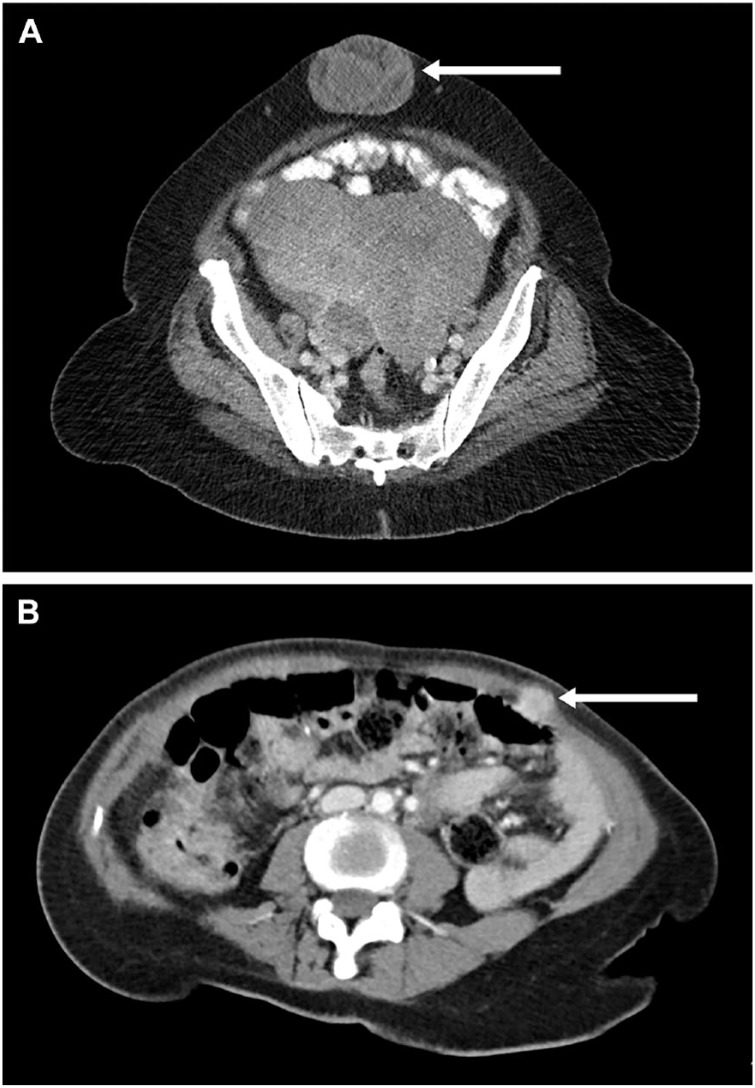
Peritoneal carcinomatosis is characterized by small, white-colored tumor depositions, which are localized on the inner surface of the visceral and parietal peritoneum (Fig. 1). Although the peritoneum is a thin and well-vascularized membrane, peritoneal metastases grow superficially, and depositions remain small. Deep invasion of the abdominal muscles and invasion of serosa of the visceral organs are rare. In fact, it is only observed after laparoscopy in the trocar opening or in case of a sister Mary Joseph nodule in the umbilicus (Fig. 2).4,5
Figure 1.
Peritoneal carcinomatosis, a condition often present in epithelial ovarian cancer, is characterized by small, white-colored tumor depositions, localized at the parietal (A–C) and visceral (D) peritoneum (arrows). The diaphragm is often involved (A, B), and presence of peritoneal carcinomatosis is generally accompanied by malignant ascites (asterisk).
Figure 2.
Extraperitoneal metastasis. (A) Sister Mary Joseph nodule. (B) Port-site metastasis in the trocar opening after a laparoscopic procedure.
The specific intra-abdominal, milliary, and superficial growth pattern of EOC metastases suggests a complex interaction between peritoneal cells and EOC cells. A complete understanding of the interactions between peritoneum and metastatic EOC at a structural and cellular level is needed to develop new treatment strategies for peritoneal carcinomatosis. Therefore, we present an overview of the literature regarding interactions between peritoneum and EOC cells that lead to the formation of peritoneal carcinomatosis, and we suggest new directions for future studies.
Peritoneal Carcinomatosis
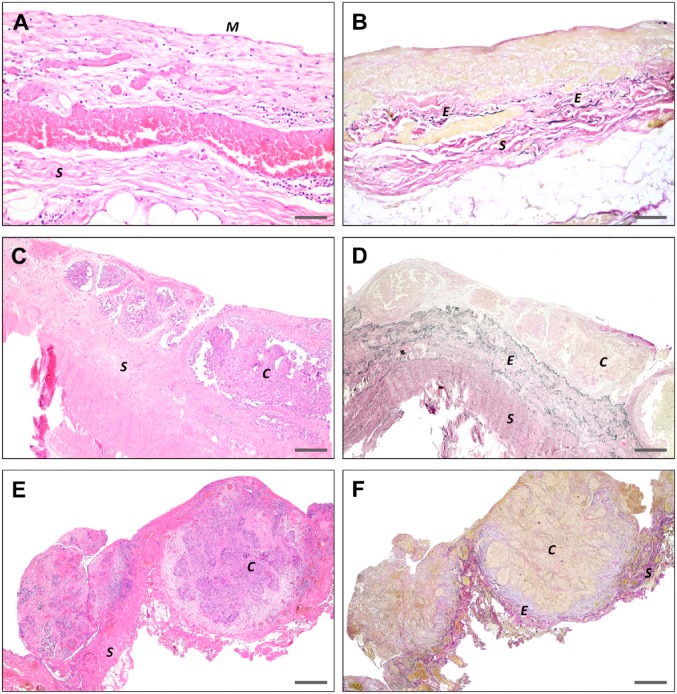
The peritoneum is a large serous organ of mesodermal origin, which exhibits both mesenchymal and epithelial characteristics. The parietal peritoneum covers the anterior and posterior abdominal walls, whereas the visceral peritoneum covers the organs. Both types of peritoneum are composed of similar distinctive layers: the glycocalyx, mesothelial cells, basal lamina, submesothelial stroma, and the elastic lamina (Figs. 3 and 4). The peritoneal composition is virtually similar throughout the whole abdomen, with only a variable thickness of the glycocalyx, submesothelial stroma, and elastic lamina, depending on movements of underlying organs and adjustments in response to either physiological or pathological conditions.
Figure 3.
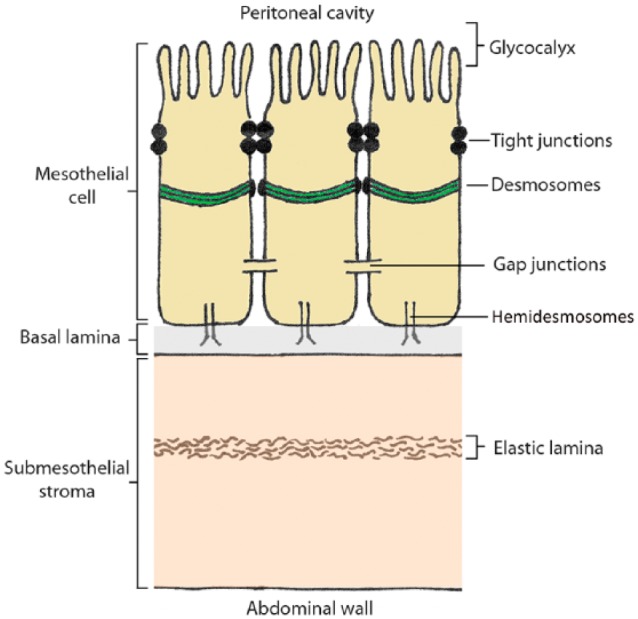
Schematic illustration of the peritoneum.
Figure 4.
Histopathological images of the peritoneum. (A) H&E staining of normal peritoneum showing mesothelial cells (M) lining the peritoneum and submesothelial stroma (S). Small areas of the mesothelial cells are denuded, due to trauma during surgery or tissue processing. (B) EVG staining showing the peritoneal elastic fibers (E), embedded in the stroma. C–F. H&E and EVG staining of peritoneal metastases show the small-sized depositions of EOC (C), with only superficial invasion of stroma (S) and absence of mesothelial cells in the presence of EOC. Scale bars A and B = 125 μm; C and D = 500 μm; E and F = 1 mm. Abbreviations: EVG, Elastic van Gieson; EOC, epithelial ovarian cancer.
The predominant role of peritoneal cells is the regulation of intraperitoneal homeostasis of the abdominal cavity by the exchange of molecules and fluids. In addition, the peritoneum plays an important role in inflammatory responses, antigen presentation, fibrosis and fibrinolysis, tissue repair, and development of peritoneal metastases. An overview of the embryology, anatomy, and physiological functions of the peritoneum was described recently by van Baal et al.6
The role of the peritoneum in relation to tumor dissemination has been studied extensively. In 1889, Paget7 launched the “seed” and “soil” theory to explain development of metastases. This theory describes the multiple interactions between cancer cells (“seeds”) and specific organ microenvironments (“soil”). In case of peritoneal carcinomatosis, this notion implies that for EOC cells, the peritoneum is their soil. The presence of peritoneal depositions, however, depends on both intrinsic characteristics of the cancer cells and responses of the peritoneum and its environment. In accordance with Paget’s theory, studies from Lyden’s group demonstrate that extracellular vesicles derived from solid malignancies, including lung, brain, liver, and pancreas malignancies, prepare the microenvironment of future metastatic sites before arrival of cancer cells, thereby creating a pre-metastatic niche.8–10 Although this has not been demonstrated yet in EOC, EOC cells release extracellular vesicles in the ascites, and it is plausible that extracellular vesicles from EOC “home” the recipient peritoneal cells by altering their microenvironment.11 Surprisingly, peritoneal carcinomatosis is often seen in the serous subtype of EOC and less frequently in other histotypes of EOC such as mucinous or endometrioid carcinomas.12 Furthermore, serous borderline (or low malignant potential) tumors are often associated with non-invasive peritoneal implants, whereas for mucinous borderline tumors, peritoneal implants do not occur. This suggests not only a specific receptive environment of the peritoneum, but also specific intrinsic characteristics of the tumor cells.
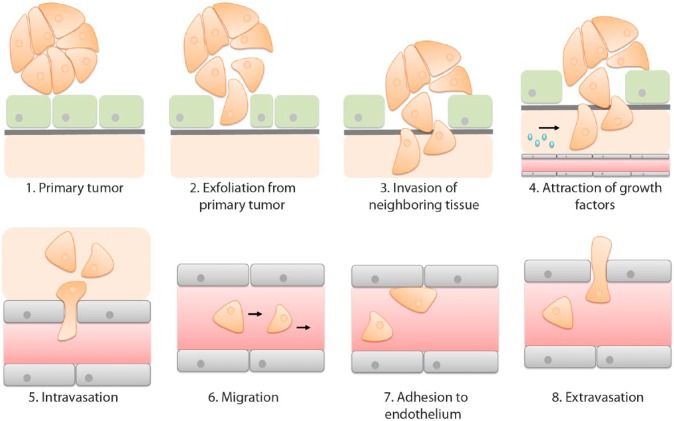
A metastasis occurs when cancer cells exfoliate from their primary origin, grow through an epithelial or endothelial cell layer, destruct the underlying basal lamina, plough through the stroma, become resistant to anoikis (apoptosis due to detachment from extracellular matrix), attach to their new “soil” and attract growth factors to further expand their growth and invasion (Fig. 5). The newly formed cluster of cancer cells induces angiogenesis and in combination with blood vessels in the surroundings of the tumor, a route for distant metastases is initiated. After intravasation, cancer cells migrate via the circulation and, once arrived at a preferred metastatic site, adhere to the endothelial lining of blood vessels. Subsequently, extravasation occurs, a process characterized by penetration of the endothelium and underlying basal lamina by cancer cells (Fig. 5).13 In peritoneal carcinomatosis, however, this process seems to be interrupted, leading to a restricted size of tumor depositions and a lack of deep invasive growth. To fully understand the specific milliary growth pattern of peritoneal metastases, knowledge is required of all sequential, interrelated steps of this multistage process.
Figure 5.
Development of metastasis of an epithelial malignancy. Metastatic cancer cells detach from a primary epithelial malignancy (1) and grow through an epithelial cell layer (2). Underlying basal lamina and stroma are penetrated by the exfoliated cancer cells (3). When resided in the stroma, cancer cells resist apoptosis and recruit growth factors that promote proliferation and angiogenesis (4). Blood vessels in the proximity of a tumor enable development of metastasis to a distant location. Cancer cells penetrate through the endothelial cell lining the blood vessels (5) and are transferred to secondary regions (6). At a future metastatic location, cancer cells adhere (7) and penetrate (8) the endothelium to develop a new metastasis.
EOC Cell Detachment From Primary Tumor
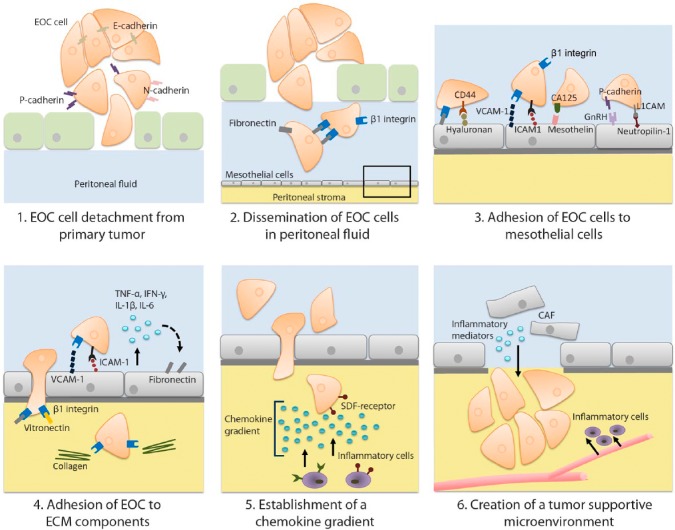
Epithelial cells in primary EOC are stationary cells with an apical-basolateral polarity, which are attached to each other via intercellular junctions (Fig. 3). To metastasize, EOC cells need to acquire a mobile and invasive phenotype (Fig. 6). Therefore, EOC cells undergo epithelial-to-mesenchymal transition (EMT).14 EOC cells express various adhesion molecules that are either up- or downregulated to obtain an invasive migratory phenotype. One group of adhesion molecules that changes its expression during EMT is the cadherins, the so-called cadherin switch. Epithelial (E)-cadherin, a glycoprotein present in desmosomes, acts as a major suppressor of cell motility, invasion, and metastasis.15 In 60% of epithelial carcinomas including EOC, expression of E-cadherin is reduced during EMT.16,17 Downregulation of E-cadherin expression contributes to detachment and subsequent formation of EOC cell clusters in the ascites (Fig. 6). Several studies demonstrated a correlation between the downregulation of E-cadherin expression on cancer cells and poor patient survival.18,19 Other cadherins, including neural (N)-cadherin and platelet (P)-cadherin, are upregulated on the surface of cancer cells during and after EMT, promoting motility, invasion, and angiogenesis. Various studies demonstrated an association between increased P-cadherin expression and depth of invasion of EOC.14 These results imply that cadherins play a role in the detachment of EOC cells from the primary tumor.
Figure 6.
Development of peritoneal metastasis of epithelial ovarian carcinoma. (1) EOC cells exfoliate from a primary tumor and undergo EMT to acquire an invasive migratory phenotype, which is characterized by a cadherin switch. (2) In the peritoneal fluid, EOC cells form tumor cell clusters via β1 integrin-fibronectin interactions that prevents anoikis. (3) EOC cells migrate passively within the peritoneal fluid along the peritoneum. To develop a peritoneal metastasis, EOC cells adhere to the mesothelial cells by integrin-mediated and non-integrin–mediated interactions. (4) Once the EOC cells have penetrated the mesothelial cell layer, EOC cells bind to components of the ECM within the stroma of the peritoneum, and an inflammatory response is generated resulting in increased production of pro-inflammatory cytokines. (5) Within the peritoneal stroma, a chemokine gradient is produced. Inflammatory cells are recruited along the gradient toward the EOC cells and contribute further to cancer progression by production of proteases, angiogenic factors, growth factors, and cytokines, which suppress immune responses. (6) To produce a tumor-supportive microenvironment, cancer-associated fibroblasts, which originate from mesothelial cells, produce cytokines and VEGF. Abbreviations: EOC, epithelial ovarian cancer; EMT, epithelial-to-mesenchymal transition; ECM, extracellular matrix; E-cadherin, epithelial cadherin; N-cadherin, neural cadherin; P-cadherin, platelet cadherin; VEGF, vascular endothelial growth factor; VCAM-1 = vascular cell adhesion molecule; ICAM-1 = intercellular cell adhesion molecule; CA125/MUC16 = Cancer Antigen 125/Mucin 16; GnRH = Gonadotropin-releasing hormone; L1CAM = L1 cell adhesion molecule; TNF-α, tissue necrosis factor-α; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin-6; SDF, stromal-derived factor; CAF, cancer-associated fibroblasts.
Dissemination Through the Abdominal Cavity
After exfoliation of EOC cells from their primary origin, the cancer cells are passively transferred via the peritoneal fluid to the peritoneum (Fig. 6). Between the visceral and parietal peritoneum, a constant volume of 5–20 mL peritoneal fluid moisturizes the peritoneum to facilitate friction-free bowel movements and which enables exchange of immune cells and other substances with plasma. The normal peritoneal fluid is a mixture of ovarian exudate, plasma transudate, tubal fluid, and retrograde menstruation.6 In the majority of patients who initially present with EOC, the balance between production and drainage of peritoneal fluid via the diaphragmatic lymphatic system is disturbed, leading to accumulation of peritoneal fluid, also called ascites. As a consequence of mechanical obstruction by tumor masses, drainage of peritoneal fluid via the lymphatic system is decreased. Increased production of peritoneal fluid in patients with EOC is most likely caused by the tumor, due to stimulation by inflammatory mediators.20 Besides cancer cells, ascites contains mesothelial cells, proteins, and various immune cells including macrophages, monocytes, natural killer cells, granulocytes, lymphocytes, eosinophils, and mast cells.21
Gravity, respiratory movements, and continuous bowel peristalsis create an intra-abdominal flow that transfers peritoneal fluid in a repetitive pattern from the lower abdomen to the upper abdomen.22 This circulation pattern is considered to be the basis of the preferred areas for metastases of EOC, such as the omentum, paracolic gutters, and the right diaphragm.
EOC cells can survive within the abdominal cavity as single cells, but clustered cancer cells are more resistant to apoptosis due to Akt kinase activation. Unlike normal cultured cells, clustered cancer cells display Akt kinase activation, which prevents apoptosis and stimulates survival through inhibition of caspase-3 activity, a crucial regulator of apoptosis.23 Furthermore, survival signaling pathways are activated by the expression of specific adhesion molecules on clustered cancer cells, such as non-ligated integrins.24
It is unknown whether clusters of tumor cells exfoliate from the primary tumor or single cells, which subsequently cluster within the abdominal cavity. A study with 3-dimensional in vitro cell cultures (spheroids) demonstrated that α5β1 integrin and its ligand fibronectin are exposed on EOC spheroids.25 Monoclonal antibodies against β1 integrin inhibited spheroid formation, suggesting an important role for β1 integrin-fibronectin interaction in tumor cell clustering in the abdominal cavity (Fig. 6). Furthermore, in vivo studies demonstrated that growth of spheroids is supported by the microenvironment of the ascites and that spheroids are less susceptible to chemotherapeutic therapy.26,27 It has been hypothesized that in patients with EOC, floating cancer cell clusters endure initial therapy, survive in ascites, and subsequently develop recurrent metastases on the peritoneal surfaces. This hypothesis may clarify the clinical observations of high recurrence rates of EOC even after complete cytoreduction and clinical chemotherapy response.
Adhesion of EOC Cells to Mesothelial Cells and Submesothelial Stroma
Various adhesion molecules are involved in the binding of EOC cells to mesothelial cells and to the underlying submesothelial stroma (Fig. 6). EOC cells expose integrins, which are glycoproteins that contribute to adhesion between EOC cells and the peritoneum. Ligands of integrins include collagen, laminin, fibronectin, fibrinogen, vitronectin, and other components of the extracellular matrix (ECM) in the stroma (Fig. 6).
Integrins are heterodimers composed of an α- and a β-subunit. On the surface of EOC cells, α5β1 integrin is abundantly expressed. This integrin binds to fibronectin exposed on mesothelial cells and thereby facilitates the first step in development of peritoneal metastases.25,28 Other integrin ligands include intercellular adhesion molecules (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1.29,30 ICAM-1 and VCAM-1 are also expressed by mesothelial cells, and their binding to integrins upregulates the production of inflammatory mediators such as tissue necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-1β and IL-6 (Fig. 6). These inflammatory mediators further stimulate the expression of fibronectin on mesothelial cells and thereby promote binding with adhesion molecules. Therefore, it is assumed that once interactions between EOC cells and peritoneum have been established, an enhanced inflammatory response leads to increased EOC cell adhesion.31,32 Cytokines such as transforming growth factor (TGF)-β1 and IL-1β induce a myofibroblastic phenotype in mesothelial cells after a mesothelial-to-mesenchymal transition (MMT).33,34 The myofibroblastic phenotype amplifies integrin-dependent EOC cell adhesion.35,36
Preclinical studies showed that anti-β1 integrin neutralizing antibodies were unable to block adhesion of EOC cells to peritoneum completely.37 Intravenous integrin-antagonism therapy for advanced EOC was tested in various clinical trials.38,39 The phase I and II trials targeting α5β1 or αvβ3 integrins with volociximab and vitaxin, respectively, showed little toxicity but also lack of clinical tumor response. The patient population in these studies consisted of refractory patients, which does not exclude that this treatment is more effective in a different stage of the disease. It was hypothesized that treatment in early stages or after cytoreductive surgery with minimal residual disease could have a greater effect on limiting the development of peritoneal carcinomatosis. Possibly, intraperitoneal administration of integrin-antagonists that showed effects in preclinical studies,15,40 or a combination of antagonists targeting multiple integrins, may lead to reduction of tumor growth. These studies suggest that also non-integrin adhesion molecules are involved in the interactions between EOC cells and mesothelial cells. Integrin-mediated and non-integrin–mediated adhesion molecules and their ligands of EOC cells and peritoneal cells are summarized in Table 1.
Table 1.
Adhesion of EOC to Mesothelial Cells. Integrin- and Non-Integrin–Regulated Adhesion of EOC to Mesothelial Cells.
| Adhesion molecules on EOC | Ligands on peritoneum | References |
|---|---|---|
| α2β1 integrin | Collagen type 1 | 41,42 |
| α3β1 integrin | Collagen type 1 Laminin |
43 |
| α4β1 integrin | VCAM-1 | 30 |
| α5β1 integrin | Fibronectin | 15,37,44 |
| α6β1 integrin | Collagen type 1 Laminin | 43 |
| αvβ1 integrin | Fibronectin | 43 |
| CD43 | ICAM-1 | 45 |
| CD44 | Hyaluronan | 28,46 |
| CA125/MUC16 | Mesothelin | 47 |
| L1CAM | Neutropilin-1 | 48 |
| GnRH | P-cadherin | 49 |
Abbreviations: EOC, epithelial ovarian cancer; VCAM-1 = vascular cell adhesion molecule; ICAM-1 = intercellular cell adhesion molecule; CA125/MUC16 = Cancer Antigen 125/Mucin 16; L1CAM = L1 cell adhesion molecule; GnRH = Gonadotropin-releasing hormone.
The process of adhesion and transmigration of thrombocytes and neutrophils through a vascular endothelial cell layer has been investigated thoroughly. Adhesion molecules and ligands, such as selectins, integrins, and ICAM-1, which are involved in this migration, have been identified precisely, each facilitating different steps in this process. Initial adhesion between neutrophils and endothelium consists of tethering and subsequent rolling.50,51 However, 90% of neutrophils that display initial adhesion with the endothelium detach from the vascular wall and continue to circulate. A minority of the attached neutrophils develop a firmer adhesion, which eventually enables transmigration through the endothelium. Although these processes have not been identified for EOC cells, it may be possible that for EOC cell interactions with the mesothelium and subsequent passage of the mesothelium, a firm adhesion following initial adhesion is also required. In line with this hypothesis, Gebauer et al.52 investigated the role of selectin binding in peritoneal metastases of pancreatic adenocarcinoma. Mesothelial cells expressed both E-selectin and P-selectin and with dynamic flow assays and flow cytometry, efficient binding of cells from a pancreatic adenocarcinoma cell line with these selectins was shown. Furthermore, E-selectin- and P-selectin-deficient mice showed significantly less peritoneal metastases compared with wild-type mice. This underlines the importance of selectins in the process of peritoneal metastases of pancreatic adenocarcinoma and suggests a comparable role in the development of peritoneal metastases of EOC.
In summary, adhesion molecules play an important role in adhesion of EOC cells to the mesothelium and underlying stroma. However, to develop successful treatment against EOC cell adhesion, multifactorial treatment should be considered because multiple adhesion molecules appear to contribute in the interactions between EOC cells and peritoneum.
Penetration of the Mesothelium by EOC Cells
The exact components of the peritoneum to which EOC cells adhere is unknown, but it has been shown that EOC cells attach more efficiently to stroma than to mesothelial cells.28,53 Therefore, an intact continuous mesothelial cell layer is considered to protect the peritoneum against adhesion and cancer spread across the peritoneum. Thus, after infection or surgery, the disrupted mesothelium possibly facilitates cancer cell adhesion by exposing its underlying stroma.54 In line with this hypothesis, it was demonstrated that mesothelial cells are absent underneath peritoneal EOC depositions.44,55 This observation supports the requirement of a mesothelial clearance before the development of a peritoneal metastatic deposition. A similar phenomenon was found in the liver where endothelial cells retracted in the presence of colon cancer cells, thus enabling adhesion of the cancer cells to hepatocytes.56
Two pathways have been described for EOC to circumvent the intact mesothelial cell layer: (1) invasion of (physiological) intercellular spaces between mesothelial cells by EOC cells57 or (2) removal or retraction of mesothelial cells.44,55 Regarding the first pathway, an example of physiological intercellular spaces is the lymphatic stomata. Stomata are small gaps between mesothelial cells with a direct connection with the lymphatic system.58 The mesothelial cells adjacent to the stomata present dehiscence of intercellular junctions. Possibly, EOC cells adhere in these areas because of the local lack of mesothelial cells and direct exposure of the submesothelial layer.57 Hypothetically, blockage of these stomata by EOC cells leads to prevention of lymph drainage and subsequent accumulation of ascites in the peritoneal cavity. Gerber et al.59 investigated peritoneal milky spots. Milky spots are immune cell aggregates that are primarily composed of lymphocytes, B-cells, and macrophages, which are often localized near lymphatic stomata. Enhanced vascularization and high levels of secreted vascular endothelial growth factor (VEGF), which are necessary for cancer cells to survive and proliferate, were observed around milky spots.60 This may clarify the further expansion across the peritoneum after adhesion of EOC to milky spots. In addition, cancer cells produce inflammatory cytokines, such as TNF-α and IL-1β,61 which are also present in ascites of patients with EOC.62 Several studies demonstrated that mesothelial cells retract in response to these inflammatory mediators, exposing the underlying stroma, which promotes cancer cell adhesion to stroma.63,64
The second theory on passage of the mesothelial cell layer was investigated by Iwanicki et al. and Kenny et al.44,55 with in vitro time-lapse video microscopy. In this study, a mesothelial cell clearance was demonstrated after attachment of EOC spheroids to mesothelial cells. The removal of mesothelial cells underneath and in the proximity of attached spheroids was directed by myosin. Myosin interacts with the actin cytoskeleton, which spans the cytoplasm. Myosin and the actin cytoskeleton together form the actomyosin complex, which can exert mechanical force within the cell to keep or change the cell shape, contract the cell, move proteins across the cell surface, and so forth. The actin cytoskeleton is via various proteins, including, for example, talin, attached to the intracellular domain of specific integrins to generate a mechanical force on the submesothelial stroma.65,66 Downregulation of myosin expression by small hairpin RNA (shRNA) and small interfering RNA (siRNA) against myosin II, but also treatment with α5 integrin blocking antibody did not affect spheroid adhesion to mesothelial cells, but prevented the subsequent migration and removal of mesothelial cells underneath tumor spheroids.44 In line with these results, Mitra et al.67 investigated EOC metastases in a xenograft model and suggested a mechanical, contractile force primarily generated by the cytoskeleton and driven by the fibronectin receptor α5β1 integrin expressed on cancer cells, which promotes invasion of the stroma.
Overall, evidence suggests that the mesothelium is a protective barrier for metastatic tumor growth and has to be circumvented by EOC cells to “seed” on the peritoneum. Although the exact mechanisms for this evasion are yet incompletely understood, it is most likely a multifactorial process that requires adaptive cellular behavior of both EOC cells and mesothelial cells.
Invasion of Submesothelial Stroma
Once the mesothelial cell layer and basal lamina are penetrated, EOC cells have access to the underlying stroma that offers a rich source of mediators enabling survival, proliferation, and invasion of EOC cells. Inflammatory cells exposing receptors for monocyte chemoattractant protein (MCP)-1 and stromal derived factor (SDF)-1 are attracted and migrate along an established chemical gradient toward the source of the chemokines.68 A receptor for SDF-1, chemokine receptor type 4 (CXCR4), is exposed by the majority of EOC cells and has been observed in the early stages of the development of EOC.69,70 Increased expression of CXCR4 on EOC, compared with CXCR4-negative EOC, is associated with more advanced disease and poor prognosis.71 Cancer cells exposing CXCR4 are attracted to SDF-1, which is primarily expressed by mesenchymal stroma cells. SDF-1 interaction with CXCR4 can initiate metastasis in mesenchymal stroma niches.72 The interaction of SDF-1 with CXCR4 is considered to contribute substantially to EOC cell invasion and formation of metastases of EOC. This assumption has initiated studies on the effects of interference with the SDF-1–CXCR4 signaling pathway on tumor growth. Various mouse model studies demonstrated a reduced growth and dissemination of peritoneal metastases of EOC and a prolonged survival after administration of AMD3100, a CXCR4 antagonist.73,74 These results imply that the SDF-1–CXCR4 axis plays an important role in the pathogenesis of EOC and peritoneal metastases. Although the efficacy of CXCR4 antagonists has not been tested in clinical trials yet, findings of preclinical studies are promising for development of new therapeutic strategies against peritoneal metastases.
Recruited inflammatory cells contribute to the production of proteases, angiogenic factors, growth factors, and immunosuppressive cytokines.61 This cascade is considered to contribute to cancer cell growth and survival (Fig. 6). Furthermore, matrix metalloproteinase (MMP)-2, MMP-9, and MMP-14 regulate destruction of stroma by proteolysis of its ECM components, and, therefore, MMPs are considered to facilitate invasion of stroma by cancer cells.75,76 Recently, it was suggested that migration and invasion of cancer cells can also occur protease-independently, through the ECM network.77 This may explain why clinical trials using orally administered MMP inhibitors demonstrated only limited efficacy, because tumor invasion then occurs without MMP activity.78 The capacities of cancer cells to migrate through the ECM network of the peritoneum may also explain growth of metastases into the port-site after disruption of the mesothelial layer and submesothelial stroma in laparoscopic procedures in EOC. Metastases of EOC may incidentally grow through the viscera of the bowel or the bladder.79 The visceral and parietal peritoneal surfaces have a similar structural composition throughout the abdomen. The incidental invasive growth in specific areas, therefore, suggests that the peritoneal microenvironment, or structures that support the peritoneum, play a role in the development of deep invasive growth.
Once clusters of cancer cells have invaded the stroma of the peritoneum, the endothelium lining the lymph vessels and blood vessels in the stroma further promotes cancer survival by recruitment of monocytes, polymorphonuclear leukocytes, lymphocytes, platelets, and probably circulating stem cells (Fig. 6).80
Thus, for a malignant tumor to develop and to metastasize, a tumor microenvironment is created that promotes tumor growth and invasion. An important step in this process is the attraction of stromal cells. Among these stromal cells, cancer-associated fibroblasts (CAF) are the most important cells that create a tumor-supportive microenvironment.81,82 Normal fibroblasts are recruited and transform under the influence of cancer cells into myofibroblastic cells that produce growth factors and ECM components that stimulate angiogenesis.81 Recently, it has been demonstrated that also in peritoneal metastases of EOC, CAFs originating from mesothelial cells play an important role in tumor progression (Fig. 6). Various studies demonstrated that after MMT, mesothelial cells can become CAFs that are able to produce cytokines and VEGF, and remodel the ECM, thereby creating a tumor-supportive environment facilitating tumor proliferation, invasion, and metastases.35,83 Presumably, the upregulated integrin-dependent cancer cell adhesion after MMT further stimulates dissemination of peritoneal metastases on the peritoneum.35,36
Milliary Growth Pattern of Peritoneal Carcinomatosis
Taken together, several factors facilitate peritoneal tumor growth and invasion. EOC cells adhere to the peritoneum, penetrate into the mesothelium, but despite the seemingly growth- and survival-stimulating microenvironment of the peritoneum, tumor invasion of the peritoneal stroma is and remains only superficial. Peritoneal or EOC characteristics that may inhibit peritoneal tumor growth and invasion have not been identified yet. Thus far, only a few studies investigated the possible aspects of the peritoneum that inhibit tumor growth and invasion.
In a study of Stadlmann et al.,84 a possible mesothelial barrier function was demonstrated via the release of TGF-β by mesothelial cells. With a co-culture model, a growth inhibition of almost 50% of EOC spheroids was observed in response to TGF-β released by mesothelial cells. However, a neutralizing antibody against TGF-β did not completely prevent growth inhibition. This suggests that mesothelial cells only partly modulate metastatic tumor growth and invasion by TGF-β secretion.
In line with these results, van der Bij et al.85 investigated the microenvironment of peritoneal carcinomatosis of colorectal carcinoma. Peritoneal resident macrophages, or M2 macrophages, activated via the classical pathway, actively limited growth of peritoneal metastases. Interestingly, a 2-fold increase of metastatic tumor growth was observed after elimination of the resident macrophages. When these findings are also applicable to EOC, a subset of macrophages may contribute to growth inhibition of peritoneal metastases of EOC.
Finally, Steinkamp et al.86 investigated intra-abdominal metastatic depositions in a mouse model and concluded that the depth of invasion is associated with the underlying peritoneal structure and the local production of chemotactic factors. It was demonstrated that cancer cells easily migrate through loosely organized tissue, such as the adipocyte-rich omentum. The cancer cells grew along blood vessels, and depth of invasion was increased by local production of chemotactic factors. In contrast, invasion of tumor depositions on dense structures such as the visceral peritoneum of the gut was restricted by smooth muscle. However, these results do not explain the occurrence of tumor growth through the visceral peritoneum of the bowels, which is observed incidentally. Neither does it explain the restricted invasion of metastases on the parietal peritoneum that is well vascularized and lacks smooth muscle. Although it is likely that differences in peritoneal microenvironment and different underlying structures facilitate or inhibit deep invasive growth of peritoneal metastases, current literature reports no conclusive data on this hypothesis.
Other Membranes and Metastases
Assuming that the peritoneum acts as a physical barrier for deep invasive growth of peritoneal metastases, it may be interesting to compare the peritoneal anatomy and functions with those of other membranes with barrier functions in the human body, such as the pleura or the blood-brain barrier. It appears that lung carcinomas and gliomas are not capable to grow directly through the pleura and blood-brain barrier, respectively, similar to the peritoneal barrier for EOC. Tight junction proteins including occludins, claudins, and zonulae occludentes of endothelial cells of the blood-brain barrier and the mesothelial cells in the pleural cavity are considered to maintain the barrier function of these compartments.87,88 These tight junctions are also present on the peritoneal mesothelial cells and possibly play a role in the superficial growth and limited size of peritoneal metastasis of EOC. However, the metastatic pattern of EOC to the peritoneum is unique and, therefore, the comparison with other membranes in the human body remains difficult.
In summary, few studies focused on the growth inhibitory functions of the peritoneum or the characteristics of the EOC that prevent further growth and invasion, and exact mechanisms are virtually unknown.
Targeted Therapy and the Peritoneum
In recent years, various targeted therapies have been developed and investigated in EOC. Bevacizumab is the first approved targeted therapy for EOC. This VEGF receptor antagonist has been approved and implemented as standard additive treatment for platinum-sensitive recurrent EOC. VEGF is a key regulator of angiogenesis, which enhances vascular permeability, stimulates endothelial cell proliferation, and enables cancer cells to sustain apoptosis.89 To support the role of VEGF in tumorigenesis, an increased expression of VEGF in EOC cells is associated with increased production of ascites and tumor progression.90
Tumor clusters are able to grow up to a size of 2 mm without external nutrients,91 but to grow further in size and to receive nutrients, tumor deposits require capillaries residing within a distance of 100 μm.92 This process is influenced by VEGF.
In a randomized controlled trial (AURELIA trial), patients with ascites at diagnosis experienced significant improvement of symptoms after the addition of bevacizumab to standard chemotherapy.93 Malignant ascites is often associated with presence of peritoneal metastases, and, therefore, these results suggest that bevacizumab also targets angiogenesis of the peritoneum.
From other targeted therapies, the exact effect on the peritoneum is unknown. Poly adenosine diphosphate ribose polymerase (PARP)-inhibitors interfere with DNA repair mechanisms. A significant prolonged progression-free survival after administration of the PARP-inhibitor olaparib in patients with platinum-sensitive recurrent EOC with either germline BRCA mutations or tumors with BRCA-like features has been shown.94 Recently, Mirza et al.95 demonstrated a significantly improved progression-free survival in patients with recurrent EOC after treatment with PARP-inhibitor niraparib, regardless of their BRCA status. However, to date, no studies described the effect of PARP-inhibitors on ascites or peritoneal disease in EOC.
Targeted therapies such as immune checkpoint inhibitors or inhibitors of the PI3K/Akt/mTOR pathway have been investigated in EOC, but no significant improvement of progression-free or overall survival has been documented.96,97
The Peritoneum as a Target for Chemotherapy
The specific intra-abdominal spreading pattern of EOC has provoked studies to investigate the benefits of intraperitoneal administration of cisplatin and paclitaxel chemotherapy in combination with intravenous administration. Intraperitoneal administration of chemotherapy resulted in a clear progression-free and overall survival benefit in patients with advanced EOC, but was associated with a high rate of toxicity and catheter-related complications.98 Patients who have minimal residual disease after cytoreductive surgery are likely to benefit most from intraperitoneal administration of chemotherapy. This may be caused by the relatively superficial penetration of intraperitoneal chemotherapy into the peritoneum. This assumption was supported by a study of Los et al.99 who analyzed intraperitoneal administration of cisplatin in a rat model. Despite the relatively high doses that were administered to rats, peritoneal penetration of cisplatin did not exceed 1–2 mm.
The promising results of the clinical trials on intraperitoneal chemotherapy have led to various trials assessing optimal dose and regimens to minimize the complications that are associated with intraperitoneal administration. The role of bevacizumab in intraperitoneal chemotherapy is currently being investigated in a large randomized controlled trial (GOG-0252). Hypothetically, bevacizumab increases peritoneal uptake of chemotherapy by reducing vascularization and subsequent interstitial fluid pressure of the peritoneal metastases. In a preclinical study of Gremonprez et al.,100 administration of bevacizumab before treatment with intraperitoneal platinum-based chemotherapy was analyzed using mouse xenograft models with peritoneal metastases of colorectal carcinoma. Compared with mice that received a placebo, mice that received bevacizumab showed a diminished interstitial fluid pressure in peritoneal metastases, which thereby enhanced the penetration and efficacy of the intraperitoneal chemotherapy and resulted in an improved local control of tumor growth. These findings on neo-adjuvant bevacizumab and intraperitoneal chemotherapy are promising for the treatment of peritoneal carcinomatosis of EOC, especially in patients with residual disease after cytoreductive surgery.
Intra-abdominal dissemination is the preferred route of metastatic EOC. The majority of patients with EOC have peritoneal carcinomatosis during initial diagnosis or recurrent disease, a condition that is associated with high morbidity and mortality. Extensive research into the pathogenesis of peritoneal carcinomatosis of EOC has been performed. Although a myriad of adhesion molecules and microenvironmental factors have been identified that may contribute to dissemination and growth of metastatic EOC, understanding of the exact mechanisms of cancer cell adhesion, the role of mesothelial cells, and factors that activate tumor growth remain largely unknown. The in appearance insignificant, thin, and elastic peritoneum is an organ with highly specialized functions. The important mechanisms how the peritoneum facilitates EOC to metastasize, but also inhibits its growth and invasion, remain to be elucidated.
Treatment options for peritoneal carcinomatosis are scarce, and therapy generally fails due to widespread peritoneal recurrences. Clearly, new treatment strategies are urgently needed, because clinical trials so far have demonstrated only limited efficacy. Integrin inhibitors may become useful in clinical practice when administered intraperitoneally or in combination with conventional chemotherapeutics and may lead to more favorable outcomes.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JOAMB performed the literature search, studied and interpreted the literature, and prepared the article. CJFN helped with the discussion and interpretation of the literature, and performed critical revision of the manuscript. RN and AS contributed to the discussion and interpretation of the literature, and performed critical revision of the manuscript. KKV contributed to the design of the article, provided intellectual contribution, and helped with critical revision of the manuscript. WJD helped with the revision of the manuscript. GGK helped with the revision of the manuscript. CARL contributed to the design of the article, to the discussion and interpretation of the literature, and performed critical revision of the manuscript. All authors approved the final manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Juliette O. A. M. van Baal, Department of Gynecologic Oncology, Center for Gynecologic Oncology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands.
Cornelis J. F. van Noorden, Cancer Center Amsterdam, Department of Medical Biology, Academic Medical Center, Amsterdam, The Netherlands.
Rienk Nieuwland, Laboratory of Experimental Clinical Chemistry, Academic Medical Center, Amsterdam, The Netherlands.
Koen K. Van de Vijver, Division of Diagnostic Oncology & Molecular Pathology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands
Auguste Sturk, Department of Clinical Chemistry, Academic Medical Center, Amsterdam, The Netherlands.
Willemien J. van Driel, Department of Gynecologic Oncology, Center for Gynecologic Oncology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands
Gemma G. Kenter, Department of Gynecologic Oncology, Center for Gynecologic Oncology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands
Christianne A. R. Lok, Department of Gynecologic Oncology, Center for Gynecologic Oncology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands
Literature Cited
- 1. Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011(8):CD007565. doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gerestein CG, Eijkemans MJ, de Jong D, van der Burg ME, Dykgraaf RH, Kooi GS, Baalbergen A, Burger CW, Ansink AC. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG. 2009;116(3):372–80. doi: 10.1111/j.1471-0528.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 3. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R; Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4. Wang PH, Yen MS, Yuan CC, Chao KC, Ng HT, Lee WL, Chao HT. Port site metastasis after laparoscopic-assisted vaginal hysterectomy for endometrial cancer: possible mechanisms and prevention. Gynecol Oncol. 1997;66(1):151–5. doi: 10.1006/gyno.1997.4717. [DOI] [PubMed] [Google Scholar]
- 5. Ching AS, Lai CW. Sonography of umbilical metastasis (Sister Mary Joseph’s nodule): from embryology to imaging. Abdom Imaging. 2002;27(6):746–9. doi: 10.1007/s00261-002-0018-2. [DOI] [PubMed] [Google Scholar]
- 6. van Baal JO, Van de Vijver KK, Nieuwland R, van Noorden CJ, van Driel WJ, Sturk A, Kenter GG, Rikkert LG, Lok CA. The histophysiology and pathophysiology of the peritoneum. Tissue Cell. 2017;49(1):95–105. doi: 10.1016/j.tice.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 7. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 8. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17(6):816–26. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng P, Yan Y, Keng S. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep. 2011;25(3):749–62. doi: 10.3892/or.2010.1119. [DOI] [PubMed] [Google Scholar]
- 12. Kobel M, Kalloger SE, Huntsman DG, Santos JL, Swenerton KD, Seidman JD, Gilks CB; Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency, Vancouver BC. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol. 2010;29(3):203–11. doi: 10.1097/PGP.0b013e3181c042b6. [DOI] [PubMed] [Google Scholar]
- 13. Martin TA, Andrew LY, Sanders J, Lane J, Jiang WG, editors. Cancer invasion and metastasis: molecular and cellular perspective. In: Jandial R, editor. Metastatic cancer: clinical and biological perspectives. Austin, Texas, Landes Bioscience; 2013;135–68. [Google Scholar]
- 14. Patel IS, Madan P, Getsios S, Bertrand MA, MacCalman CD. Cadherin switching in ovarian cancer progression. Int J Cancer. 2003;106(2):172–7. doi: 10.1002/ijc.11086. [DOI] [PubMed] [Google Scholar]
- 15. Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68(7):2329–39. doi: 10.1158/0008-5472.can-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joo YE, Rew JS, Park CS, Kim SJ. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology. 2002;2(2):129–37. doi: 10.1159/000055903. [DOI] [PubMed] [Google Scholar]
- 17. Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103(8):1631–43. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 18. Mise BP, Telesmanic VD, Tomic S, Sundov D, Capkun V, Vrdoljak E. Correlation between E-cadherin immunoexpression and efficacy of first line platinum-based chemotherapy in advanced high grade serous ovarian cancer. Pathol Oncol Res. 2015;21(2):347–56. doi: 10.1007/s12253-014-9827-1. [DOI] [PubMed] [Google Scholar]
- 19. Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktas M, Hwang MS, Darling DS, Coleman IM, Nelson PS, Nguyen HM, Corey E, Tewari M, Morrissey C, Vessella RL, Knudsen BS. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol. 2011;179(1):400–10. doi: 10.1016/j.ajpath.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyoshi A, Miyatake T, Hara T, Tanaka A, Komura N, Komiya S, Kanao S, Takeda M, Mimura M, Nagamatsu M, Yokoi T. Etiology of ascites and pleural effusion associated with ovarian tumors: literature review and case reports of three ovarian tumors presenting with massive ascites, but without peritoneal dissemination. Case Rep Obstet Gynecol. 2015;2015:414019. doi: 10.1155/2015/414019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13(4):273–82. doi: 10.1038/nrc3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raptopoulos V, Gourtsoyiannis N. Peritoneal carcinomatosis. Eur Radiol. 2001;11(11):2195–206. doi: 10.1007/s003300100998. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256. doi: 10.3389/fonc.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carduner L, Picot CR, Leroy-Dudal J, Blay L, Kellouche S, Carreiras F. Cell cycle arrest or survival signaling through alphav integrins, activation of PKC and ERK1/2 lead to anoikis resistance of ovarian cancer spheroids. Exp Cell Res. 2014;320(2):329–42. doi: 10.1016/j.yexcr.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 25. Casey RC, Burleson KM, Skubitz KM, Pambuccian SE, Oegema TR, Jr, Ruff LE, Skubitz AP. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001;159(6):2071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burleson KM, Boente MP, Pambuccian SE, Skubitz AP. Disaggregation and invasion of ovarian carcinoma ascites spheroids. J Transl Med. 2006;4:6. doi: 10.1186/1479-5876-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. L’Esperance S, Bachvarova M, Tetu B, Mes-Masson AM, Bachvarov D. Global gene expression analysis of early response to chemotherapy treatment in ovarian cancer spheroids. BMC Genomics. 2008;9:99. doi: 10.1186/1471-2164-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Jr, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93(1):170–81. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 29. Alkhamesi NA, Ziprin P, Pfistermuller K, Peck DH, Darzi AW. ICAM-1 mediated peritoneal carcinomatosis, a target for therapeutic intervention. Clin Exp Metastasis. 2005;22(6):449–59. doi: 10.1007/s10585-005-2893-8. [DOI] [PubMed] [Google Scholar]
- 30. Slack-Davis JK, Atkins KA, Harrer C, Hershey ED, Conaway M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009;69(4):1469–76. doi: 10.1158/0008-5472.can-08-2678. [DOI] [PubMed] [Google Scholar]
- 31. Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL, Ladanyi A, Penicka CV, George J, Matlin K, Montag A, Wroblewski K, Yamada SD, Mazar AP, Bowtell D, Lengyel E. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest. 2014;124(10):4614–28. doi: 10.1172/JCI74778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klein CL, Bittinger F, Skarke CC, Wagner M, Kohler H, Walgenbach S, Kirkpatrick CJ. Effects of cytokines on the expression of cell adhesion molecules by cultured human omental mesothelial cells. Pathobiology. 1995;63(4):204–12. [DOI] [PubMed] [Google Scholar]
- 33. Yanez-Mo M, Lara-Pezzi E, Selgas R, Ramirez-Huesca M, Dominguez-Jimenez C, Jimenez-Heffernan JA, Aguilera A, Sánchez-Tomero JA, Bajo MA, Alvarez V, Castro MA, del Peso G, Cirujeda A, Gamallo C, Sánchez-Madrid F, López-Cabrera M. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348(5):403–13. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- 34. Yang WS, Kim BS, Lee SK, Park JS, Kim SB. Interleukin-1beta stimulates the production of extracellular matrix in cultured human peritoneal mesothelial cells. Perit Dial Int. 1999;19(3):211–20. [PubMed] [Google Scholar]
- 35. Sandoval P, Jimenez-Heffernan JA, Rynne-Vidal A, Perez-Lozano ML, Gilsanz A, Ruiz-Carpio V, Reyes R, García-Bordas J, Stamatakis K, Dotor J, Majano PL, Fresno M, Cabañas C, López-Cabrera M. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J Pathol. 2013;231(4):517–31. doi: 10.1002/path.4281. [DOI] [PubMed] [Google Scholar]
- 36. Jiang CG, Lv L, Liu FR, Wang ZN, Na D, Li F, Li JB, Sun Z, Xu HM. Connective tissue growth factor is a positive regulator of epithelial-mesenchymal transition and promotes the adhesion with gastric cancer cells in human peritoneal mesothelial cells. Cytokine. 2013;61(1):173–80. doi: 10.1016/j.cyto.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 37. Strobel T, Cannistra SA. Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73(3):362–7. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- 38. Bell-McGuinn KM, Matthews CM, Ho SN, Barve M, Gilbert L, Penson RT, Lengyel E, Palaparthy R, Gilder K, Vassos A, McAuliffe W, Weymer S, Barton J, Schilder RJ. A phase II, single-arm study of the anti-alpha5beta1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol Oncol. 2011;121(2):273–9. doi: 10.1016/j.ygyno.2010.12.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Posey JA, Khazaeli MB, DelGrosso A, Saleh MN, Lin CY, Huse W, LoBuglio AF. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm. 2001;16(2):125–32. doi: 10.1089/108497801300189218. [DOI] [PubMed] [Google Scholar]
- 40. Landen CN, Kim TJ, Lin YG, Merritt WM, Kamat AA, Han LY, Spannuth WA, Nick AM, Jennnings NB, Kinch MS, Tice D, Sood AK. Tumor-selective response to antibody-mediated targeting of alphavbeta3 integrin in ovarian cancer. Neoplasia. 2008;10(11):1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moser TL, Pizzo SV, Bafetti LM, Fishman DA, Stack MS. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the alpha2beta1 integrin. Int J Cancer. 1996;67(5):695–701. doi: [DOI] [PubMed] [Google Scholar]
- 42. Fishman DA, Kearns A, Chilukuri K, Bafetti LM, O’Toole EA, Georgacopoulos J, Ravosa MJ, Stack MS. Metastatic dissemination of human ovarian epithelial carcinoma is promoted by alpha2beta1-integrin-mediated interaction with type I collagen. Invasion Metastasis. 1998;18(1):15–26. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed N, Riley C, Rice G, Quinn M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis. 2005;22(5):391–402. doi: 10.1007/s10585-005-1262-y. [DOI] [PubMed] [Google Scholar]
- 44. Iwanicki MP, Davidowitz RA, Ng MR, Besser A, Muranen T, Merritt M, Danuser G, Ince TA, Brugge JS. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;1(2):144–57. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ziprin P, Alkhamesi NA, Ridgway PF, Peck DH, Darzi AW. Tumour-expressed CD43 (sialophorin) mediates tumourmesothelial cell adhesion. Biol Chem. 2004;385(8):755–61. doi: 10.1515/BC.2004.092. [DOI] [PubMed] [Google Scholar]
- 46. Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154(5):1525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279(10):9190–8. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 48. Stoeck A, Schlich S, Issa Y, Gschwend V, Wenger T, Herr I, Marmé A, Bourbie S, Altevogt P, Gutwein P. L1 on ovarian carcinoma cells is a binding partner for Neuropilin-1 on mesothelial cells. Cancer Lett. 2006;239(2):212–26. doi: 10.1016/j.canlet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 49. Cheung LW, Yung S, Chan TM, Leung PC, Wong AS. Targeting gonadotropin-releasing hormone receptor inhibits the early step of ovarian cancer metastasis by modulating tumor-mesothelial adhesion. Mol Ther. 2013;21(1):78–90. doi: 10.1038/mt.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100(12):1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 51. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 52. Gebauer F, Wicklein D, Stubke K, Nehmann N, Schmidt A, Salamon J, Peldschus K, Nentwich MF, Adam G, Tolstonog G, Bockhorn M, Izbicki JR, Wagener C, Schumacher U. Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp–/rag2– mice. Gut. 2013;62(5):741–50. doi: 10.1136/gutjnl-2011-300629. [DOI] [PubMed] [Google Scholar]
- 53. Niedbala MJ, Crickard K, Bernacki RJ. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Res. 1985;160(2):499–513. [DOI] [PubMed] [Google Scholar]
- 54. Mutsaers SE. The mesothelial cell. Int J Biochem Cell Biol. 2004;36(1):9–16. [DOI] [PubMed] [Google Scholar]
- 55. Kenny HA, Nieman KM, Mitra AK, Lengyel E. The first line of intra-abdominal metastatic attack: breaching the mesothelial cell layer. Cancer Discov. 2011;1(2):100–2. doi: 10.1158/2159-8290.CD-11-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mook OR, van Marle J, Jonges R, Vreeling-Sindelarova H, Frederiks WM, Van Noorden CJ. Interactions between colon cancer cells and hepatocytes in rats in relation to metastasis. J Cell Mol Med. 2008;12(5B):2052–61. doi: 10.1111/j.1582-4934.2008.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clark R, Krishnan V, Schoof M, Rodriguez I, Theriault B, Chekmareva M, Rinker-Schaeffer C. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am J Pathol. 2013;183(2):576–91. doi: 10.1016/j.ajpath.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang ZB, Li M, Li JC. Recent advances in the research of lymphatic stomata. Anat Rec. 2010;293(5):754–61. doi: 10.1002/ar.21101. [DOI] [PubMed] [Google Scholar]
- 59. Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, Lord EM. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169(5):1739–52. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev. 2012;31(1–2):143–62. doi: 10.1007/s10555-011-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 62. Kutteh WH, Kutteh CC. Quantitation of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in the effusions of ovarian epithelial neoplasms. Am J Obstet Gynecol. 1992;167(6):1864–9. [DOI] [PubMed] [Google Scholar]
- 63. Yonemura Y, Endou Y, Nojima M, Kawamura T, Fujita H, Kaji M, Ajisaka H, Bandou E, Sasaki T, Yamaguchi T, Harada S, Yamamoto H. A possible role of cytokines in the formation of peritoneal dissemination. Int J Oncol. 1997;11(2):349–58. [DOI] [PubMed] [Google Scholar]
- 64. Stadlmann S, Raffeiner R, Amberger A, Margreiter R, Zeimet AG, Abendstein B, Moser PL, Mikuz G, Klosterhalfen B, Offner FA. Disruption of the integrity of human peritoneal mesothelium by interleukin-1beta and tumor necrosis factor-alpha. Virchows Arch. 2003;443(5):678–85. doi: 10.1007/s00428-003-0867-2. [DOI] [PubMed] [Google Scholar]
- 65. Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 66. Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10(9):1062–8. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30(13):1566–76. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 69. Archibald KM, Kulbe H, Kwong J, Chakravarty P, Temple J, Chaplin T, Flak MB, McNeish IA, Deen S, Brenton JD, Young BD, Balkwill F. Sequential genetic change at the TP53 and chemokine receptor CXCR4 locus during transformation of human ovarian surface epithelium. Oncogene. 2012;31(48):4987–95. doi: 10.1038/onc.2011.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rezaeifard S, Razmkhah M, Robati M, Momtahan M, Ghaderi A. Cytokines, chemokines, and chemokine receptors quantitative expressions in patients with ovarian cancer. Iran J Med Sci. 2015;40(3):225–32. [PMC free article] [PubMed] [Google Scholar]
- 71. Guo L, Cui ZM, Zhang J, Huang Y. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chin J Cancer. 2011;30(5):336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35(7):816–26. doi: 10.1038/onc.2015.139. [DOI] [PubMed] [Google Scholar]
- 73. Righi E, Kashiwagi S, Yuan J, Santosuosso M, Leblanc P, Ingraham R, Forbes B, Edelblute B, Collette B, Xing D, Kowalski M, Mingari MC, Vianello F, Birrer M, Orsulic S, Dranoff G, Poznansky MC. CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Res. 2011;71(16):5522–34. doi: 10.1158/0008-5472.can-10-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122(1):91–9. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 75. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 76. Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705(2):69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 77. Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21(12):736–44. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 78. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 79. Cerci ZC, Sakarya DK, Yetimalar MH, Bezircioglu I, Kasap B, Baser E, Yucel K. Computed tomography as a predictor of the extent of the disease and surgical outcomes in ovarian cancer. Ginekol Pol. 2016;87(5):326–32. [DOI] [PubMed] [Google Scholar]
- 80. Kulbe H, Levinson NR, Balkwill F, Wilson JL. The chemokine network in cancer—much more than directing cell movement. Int J Dev Biol. 2004;48(5–6):489–96. doi: 10.1387/ijdb.041814hk. [DOI] [PubMed] [Google Scholar]
- 81. Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 82. Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 83. Rynne-Vidal A, Jimenez-Heffernan JA, Fernandez-Chacon C, Lopez-Cabrera M, Sandoval P. The mesothelial origin of carcinoma associated-fibroblasts in peritoneal metastasis. Cancers. 2015;7(4):1994–2011. doi: 10.3390/cancers7040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stadlmann S, Feichtinger H, Mikuz G, Marth C, Zeimet AG, Herold M, Knabbe C, Offner FA. Interactions of human peritoneal mesothelial cells with serous ovarian cancer cell spheroids—evidence for a mechanical and paracrine barrier function of the peritoneal mesothelium. Int J Gynecol Pathol. 2014;24(2):192–200. doi: 10.1097/IGC.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 85. van der Bij GJ, Bogels M, Oosterling SJ, Kroon J, Schuckmann DT, de Vries HE, Meijer S, Beelen RH, van Egmond M. Tumor infiltrating macrophages reduce development of peritoneal colorectal carcinoma metastases. Cancer Lett. 2008;262(1):77–86. doi: 10.1016/j.canlet.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 86. Steinkamp MP, Winner KK, Davies S, Muller C, Zhang Y, Hoffman RM, Shirinifard A, Moses M, Jiang Y, Wilson BS. Ovarian tumor attachment, invasion, and vascularization reflect unique microenvironments in the peritoneum: insights from xenograft and mathematical models. Front Oncol. 2013;3:97. doi: 10.3389/fonc.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dubois LG, Campanati L, Righy C, D’Andrea-Meira I, Spohr TC, Porto-Carreiro I, Pereira CM, Balça-Silva J, Kahn SA, DosSantos MF, Oliveira Mde A, Ximenes-da-Silva A, Lopes MC, Faveret E, Gasparetto EL, Moura-Neto V. Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci. 2014;8:418. doi: 10.3389/fncel.2014.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Markov AG, Amasheh S. Tight junction physiology of pleural mesothelium. Front Physiol. 2014;5:221. doi: 10.3389/fphys.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray-Coquard I. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 90. Graybill W, Sood AK, Monk BJ, Coleman RL. State of the science: emerging therapeutic strategies for targeting angiogenesis in ovarian cancer. Gynecol Oncol. 2015;138(2):223–6. doi: 10.1016/j.ygyno.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 91. Brooks PC. Cell adhesion molecules in angiogenesis. Cancer Metastasis Rev. 1996;15(2):187–94. [DOI] [PubMed] [Google Scholar]
- 92. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. [DOI] [PubMed] [Google Scholar]
- 93. Stockler MR, Hilpert F, Friedlander M, King MT, Wenzel L, Lee CK, Joly F, de Gregorio N, Arranz JA, Mirza MR, Sorio R, Freudensprung U, Sneller V, Hales G, Pujade-Lauraine E. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32(13):1309–16. doi: 10.1200/jco.2013.51.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Konecny GE, Kristeleit RS. PARP inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: current practice and future directions. Br J Cancer. 2016;115(10):1157–73. doi: 10.1038/bjc.2016.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, Christensen RD, Berek JS, Dørum A, Tinker AV, du Bois A, González-Martín A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 96. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–22. doi: 10.1200/jco.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 97. Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, Clarke PA, Raynaud FI, Levy G, Ware JA, Mazina K, Lin R, Wu J, Fredrickson J, Spoerke JM, Lackner MR, Yan Y, Friedman LS, Kaye SB, Derynck MK, Workman P, de Bono JS. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21(1):77–86. doi: 10.1158/1078-0432.ccr-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016(1):CD005340. doi: 10.1002/14651858.CD005340.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Los G, Nagel JD, McVie JG. Anti-tumor effect of cisplatin, carboplatin, mitoxantrone, and doxorubicin on peritoneal tumor growth after intraperitoneal and intravenous chemotherapy: a comparative study. Sel Cancer Ther. 1990;6(2):73–82. [DOI] [PubMed] [Google Scholar]
- 100. Gremonprez F, Descamps B, Izmer A, Vanhove C, Vanhaecke F, De Wever O, Ceelen W. Pretreatment with VEGF(R)-inhibitors reduces interstitial fluid pressure, increases intraperitoneal chemotherapy drug penetration, and impedes tumor growth in a mouse colorectal carcinomatosis model. Oncotarget. 2015;6(30):29889–900. doi: 10.18632/oncotarget.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]