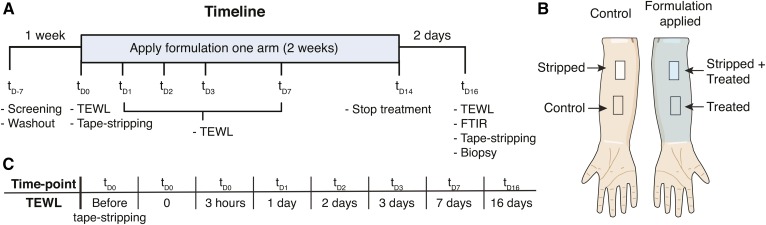
Fig. 1.
Study design. A: Timeline of the clinical study (Time points are indicated as t with days as subscripts). Volunteers were first screened by a dermatologist (tD-7), followed by a 1-week washout period. At tD0 the SC was removed by tape-stripping. The formulation was applied two times daily for 2 weeks followed by 2 days washout. The activities and measurements are indicated at each time point (tD-7 to tD16). At all indicated time points, TEWL was measured, except for tD-7. B: The studied sites on the ventral forearms. On both arms, one site was tape-stripped; on one arm, formulation was applied. C: The time points at which TEWL was measured to monitor barrier recovery of the stripped sites.