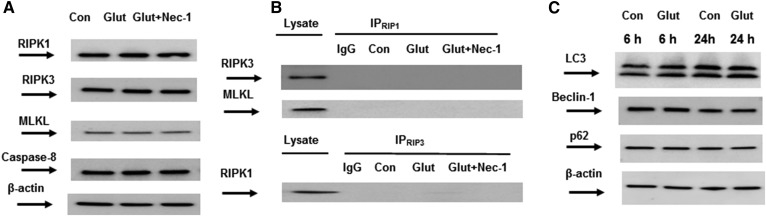
Fig. 2.
OL demise in response to glutamate is not mediated by necroptotic or autophagic signaling machinery. A: OLs were exposed to 1 mM glutamate (Glut) with/without 40 μM necrostatin-1 (Nec-1) for 24 h and cell lysates were analyzed by Western blotting using anti-RIPK1 (Cell Signaling Technology), anti-RIPK3 (Cell Signaling Technology), anti-MLKL (Thermo Fisher), and anti-caspase-8 (Cell Signaling Technology) antibodies. To confirm equal loading of samples, the membranes were stripped and probed with anti-β-actin (Sigma-Aldrich) antibody. Data are representative of three independent experiments. Con, control. B: Necrosome complex formation was probed in immunoprecipitation experiments. OLs were treated with 1 mM glutamate (Glut) with/without 40 μM necrostatin-1 (Nec-1) for 24 h. Cell lysates were immunoprecipitated with anti-RIPK1 antibodies (R&D Systems) and probed using anti-RIPK3 (Cell Signaling Technology) or anti-MLKL (Thermo Fisher) antibodies. In reciprocal experiments, cell lysates were immunoprecipitated with anti-RIPK3 antibodies (Cell Signaling Technology) and probed using anti-RIPK1 antibodies (R&D Systems). Input load: 20 μg/lane. As a control, the same immunoprecipitation procedure was performed except for primary antibody application (IgG). C: OLs were exposed to 1 mM glutamate (Glut) for 6 h or 24 h and the cell lysates’ (20 μg/lane) expression of autophagy markers was assessed by Western blotting using anti-LC3A (Cell Signaling Technology), anti-beclin-1 (Cell Signaling Technology), and anti-p62(Cell Signaling Technology) antibodies. To confirm equal loading of samples, the membranes were stripped and probed with anti-β-actin (Sigma-Aldrich) antibody. Data are representative of three independent experiments.