Abstract
N 6-methyladenosine (m6A) is the most prevalent post-transcriptional modification of eukaryotic mRNA and long noncoding RNA. m6A mediates its effects primarily by recruiting proteins, including the multiprotein eukaryotic initiation factor 3 (eIF3) complex and a set of proteins that contain the YTH domain. Here we describe the mechanisms by which YTH domain-containing proteins bind m6A and influence the fate of m6A-containing RNA in mammalian cells. We discuss the diverse, and occasionally contradictory, functions ascribed to these proteins and the emerging concepts that are influencing our understanding of these proteins and their effects on the epitranscriptome.
Keywords: N6-methyl adenosine, m6A modification, YTH proteins, RNA metabolism, translational regulation, splicing
Main text
m6A and the epitranscriptomic code
N6-methyladenosine (m6A) is the most prevalent post-transcriptional modification in eukaryotic RNAs (Box 1). It was first discovered and proposed to be a potential regulator of mRNA processing in cells in the 1970s [1, 2]. Subsequent studies in yeast showed that m6A levels are dynamic, e.g. induced upon yeast sporulation, and deletion of the m6A-synthesizing enzyme inhibited sporulation in yeast and arrested seed development [3, 4]. Thus, m6A was observed to be dynamic and functionally relevant nucleotide modification. However, m6A could only be measured in poly(A) RNA preparations, and not individual transcripts, which prevented an understanding of whether m6A was selectively located on specific mRNAs or if it was a nonspecific mark in the transcriptome. The development of epitranscriptomic mapping methods for m6A [5, 6] provided the critical technologies needed to specifically link m6A to specific mRNAs and to address the hypotheses that m6A is a regulator of mRNA biology that was initially put forward more than 40 years ago.
Box 1. m6A writers, readers, and erasers.
Adenosines in cellular RNAs can be chemically modified by the addition of a methyl group at the N6 position of the adenine base generating N6-methyladenosine (m6A). The m6A writer complex is a multi-protein complex composed of METTL3, METTL14, WTAP, and RBM15/RBM15B [16, 63, 82, 83]. METTL3 is the catalytic subunit that transfers a methyl group from S-adenosylmethionine (SAM) to an adenosine in RNA [84, 85]. m6A is found in a DRACH consensus sequence where D = A/G/U, R = A/G, and H = A/C/U. m6A can be removed by ALKBH5, which is found predominantly in the nucleus and is the only known m6A ‘eraser’ [86]. Multiple m6A reader proteins exist in both the nucleus and cytoplasm. Of the known m6A reader proteins most contain a YTH domain that specifically recognizes m6A versus A. The m6A reader YTHDC1 is predominantly found in the nucleus while YTHDF1, YTHDF2, YTHDF3, and YTHDC2 are primarily cytoplasmic. In addition to YTH proteins, eukaryotic initiation factor 3 (eIF3) serves as a reader of m6A and promotes cap-independent translation upon induction of cellular stress[18].
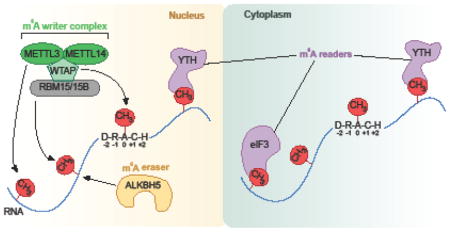
m6A has many functions in cells, with cell differentiation showing the clearest dependence on m6A [7–9]. m6A affects cancer progression [10, 11], circadian rhythm [12], neuronal function and sex determination in Drosophila [13–15], and X chromosome inactivation [16]. m6A mediates its physiologic effects by influencing the fate of mRNA in cells. One function of m6A is to destabilize local RNA structure [17] and thereby increase the accessibility of proteins to binding sites adjacent to m6A. A more influential effect of m6A is to recruit specific proteins to mRNA. m6A recruits the translation initiation factor eIF3 [18] and proteins containing a YTH (“YT521-B homology”) domain [6, 19]. m6A-dependent recruitment of eIF3 enables mRNAs to be translated in the absence of eIF4E [18], the canonical cap-binding protein. This interaction is limited to mRNAs with m6A in the 5′UTR, and may be particularly important in stress or disease states in which eIF4E is inhibited [20]. It remains unknown exactly how eIF3 recognizes m6A and which protein domains are necessary for this interaction to occur. In contrast to eIF3, YTH proteins recognize m6A through a specific well-characterized YTH domain. Here we review the diverse functions of YTH domain-containing proteins in mediating the effects of m6A in mRNA.
Diversity of YTH domain-containing proteins
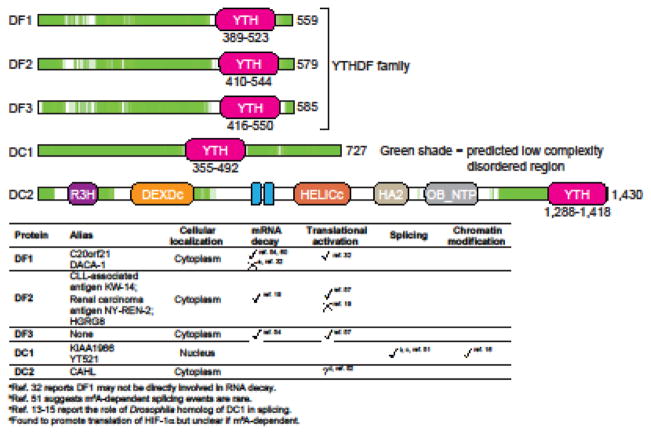
The first YTH protein was discovered in a yeast-two hybrid screen for binding partners of TRA-2β, a spliceosomal complex component [21, 22]. The recovered protein was designated YT521-B. The YTH domain was discovered in a BLAST analysis that revealed a ~140 amino acid domain that was especially highly conserved among YT521-B homologs [23]. BLAST searches using just this domain revealed YT521-B homologs as well as other proteins that contained this domain. Proteins containing this YT521-B homology or YTH domain were observed across various organisms, including plants and yeast. The YTH domain was predicted to be a novel RNA-binding domain based on homology with the RNA-binding RRM (RNA recognition motif) domain [23]. Vertebrate YTH domain-containing proteins can be classified into three categories: YTHDF (YTH domain-containing family protein) family, YTHDC1 (YTH domain-containing protein 1, also called DC1), and YTHDC2 (YTH domain-containing protein 2, also called DC2) (Figure 1). Aside from their conserved YTH domain, DC1 and DC2 are unrelated to other YTH domain-containing proteins based on amino acid sequence, size, and overall domain organization. Therefore, DC1 and DC2 are not paralogs and do not comprise a family despite their name. In contrast, the YTHDF family comprises three paralogs that share high amino acid identity over their entire length: YTHDF1, YTHDF2, and YTHDF3 (also called DF1, DF2, and DF3, respectively) (Figure 2). DF proteins possess a C-terminal YTH domain and a ~350 amino acids low complexity region, which lacks a recognizable modular protein domain and contains several P/Q/N-rich (see glossary) patches (Figure 1).
Figure 1. Domain structure and functions of human YTH proteins.
Schematic representation of domains and disordered regions of human YTH proteins: DF1, DF2, DF3, DC1, and DC2. DF1, DF2, and DF3 together make the YTHDF (DF) family. DC2 and the DF family proteins have a C-terminal YTH domain (pink), while DC1 has an internal YTH domain. All the proteins have low-complexity disordered regions (green). Disordered regions were identified using D2P2 database tools [80]. Additional domains in DC2 include R3H, DEXDc, ankyrin repeats (ANK), HELICc, HA2 and OB-fold domains. Protein length (in amino acids) is indicated next to each protein schematic. Functions of the human YTH proteins along with their alias names and major cellular localization are also indicated in a table (bottom panel).
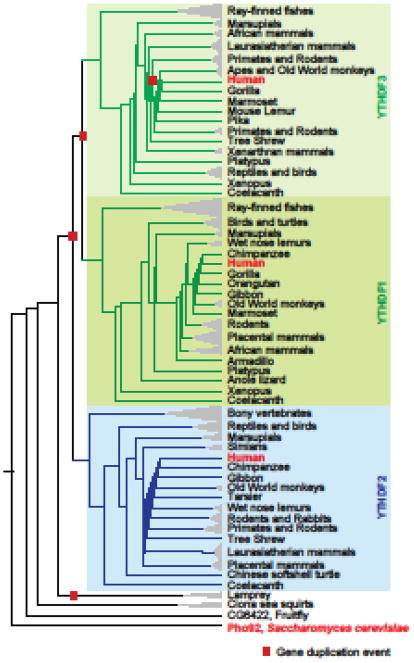
Figure 2. Human YTHDF proteins are paralogs of each other.
Shown is a species-aware phylogenetic gene tree of YTHDF family genes across the indicated organisms or group of organisms. This tree was constructed using the Ensembl Gene orthology and paralogy prediction pipeline [81]. Gene duplication events (
 ) and YTHDF1, YTHDF2, and YTHDF3 gene clades are also indicated.
) and YTHDF1, YTHDF2, and YTHDF3 gene clades are also indicated.
Compared to vertebrates, Drosophila has one homolog of the DF family and one homolog of DC1. C. elegans completely lacks YTH proteins, while Arabidopsis has 12 genes that encode YTH domain-containing proteins. Outside the YTH domains, these Arabidopsis proteins lack homology with human YTH proteins. Due to differences in the types of proteins that contain YTH domains in these organisms, the fate of m6A-containing mRNA may be different between them.
Recent studies have also examined DC2 homologs across species [24]. Although DC2 orthologs are readily detected in multicellular organisms, several organisms have DC2 orthologs in which the YTH domain was lost. In mammals, DC2 proteins contain a YTH domain except for the platypus DC2 ortholog. Similarly, some birds, amphibians and reptiles do not contain a YTH domain in their DC2 homologs [24]. Drosophila and nematodes also lack a YTH domain in their DC2 orthologs. Taken together, these data suggest that DC2 evolved to function in a YTH-independent manner in some organisms.
YTH domains: Some bind m6A, some do not
In vitro assays [6] and subsequent binding experiments using recombinantly expressed YTH domain demonstrated that the YTH domain binds RNA in an m6A-dependent manner, albeit with low affinity [25–27]. YTH domains have been reported to bind to m6A with affinities between 100 nM and 300 nM [25, 28–30]; however, some studies have also reported affinities between 1–3 μM [26, 27]. Nonmethylated RNAs typically show ~5–10 fold weaker binding. Unlike most specific RNA-binding interactions, which have affinities in the low nanomolar range [31], the weak binding affinity suggests that the YTH domain does not form a stable complex with an mRNA target on its own but requires additional interactions by their low complexity region to bind. Below we describe YTH domain interactions with m6A in mRNA.
m6A recognition by the YTH domain
YTH domains in DF proteins and DC1 employ a conserved mechanism for recognizing m6A. The structure of a YTH domain bound to m6A was described in three independent reports [25, 28, 29]. One of the reported structures was of MRB1 (methylated RNA-binding protein 1), a Zygosacchomyces rouxii YTH domain-containing protein [25]. Two other studies reported the structure of human DC1 in complex with an m6A-containing RNA [28, 29]. Structures of DC1 [28, 30], DF2 [26, 27] and DF1 [30] have since been reported.
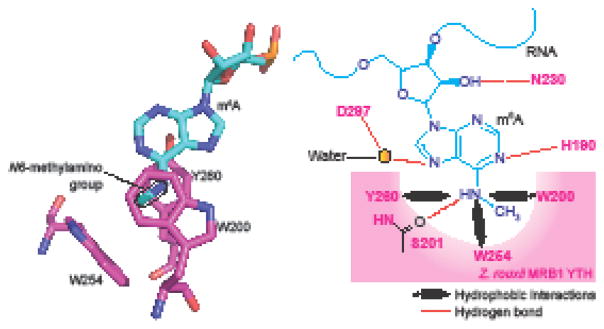
In MRB1, the methyl moiety of m6A is recognized by an aromatic cage formed from a tyrosine and two tryptophans (Figure 3) [25]. The adenine base does not form stacking interactions with any of the aromatic amino acids forming the cage. The DF1 and DF2 crystal structures show an aromatic cage comprising three tryptophans similarly recognizing the N6-methyl group (Figure 3). The cage-forming amino acids are highly conserved in YTH domains across various organisms (Figure 4). Methyl recognition by the YTH domain is different than methyl recognition by methyl-lysine-binding proteins, such as tudor and chromodomains [32, 33]. Methyl-lysine recognition involves the methylammonium (i.e., positively charged methyl-lysine side chain) forming a cation-pi interactions with an aromatic cage [34]. However, m6A is not cationic. Thus, m6A is unlikely to have the same binding strength as methyl-lysine to its cognate binding protein. Indeed, HP1 chromo domains show >10-fold increased binding selectivity towards the methylated lysine [35], while m6A binds with between 5–10-fold selectivity over the nonmethylated base.
Figure 3. A hydrophobic aromatic cage in the YTH domain recognizes m6A.
Shown here is the organization of m6A (blue) in the WWY-type aromatic cage (pink) of Z. rouxii MRB1 YTH domain. A tyrosine (Y260) and tryptophan (W200) residues in the protein sandwich the 6-methyladenine group. This positions the methyl group pointing to another tryptophan (W254) which forms the base of the cage. Notably, the purine ring of the m6A residue is not involved in this hydrophobic interaction. The amino acid positions in the domain are highly conserved across species. The YTHDF family proteins of proteins have a WWW cage, and YTHDC1 and YTHDC2 have a WWL-type cage.
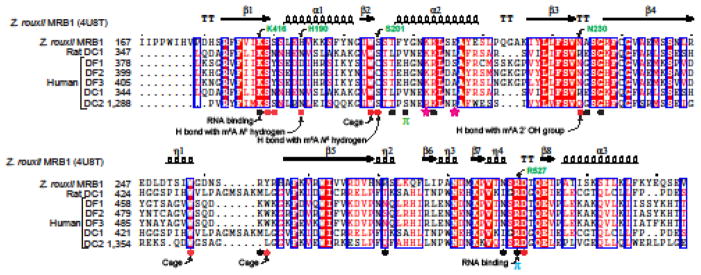
Figure 4. YTH domains share a conserved mode of binding m6A RNA.
Amino acid sequence alignment of the YTH domains from the indicated organisms is shown here. Secondary structural elements in the YTH domain from Z. rouxii MRB1 (PDB: 4U8T) are shown above the sequence alignment. Amino acid positions in individual protein are also indicated next to the protein names. Amino acids participating in recognition of different parts of m6A RNA are indicated with filled shapes below the sequence alignment. Above the alignments: TT, strict β-turns; arrows, β-strands; coils, α-helices. In the alignments: red shade with white characters, identical amino acids; red characters, similar amino acids; blue frame, marks block of sequence with similarity. Below the alignments: (
 ) Residues making contact with bases other than m6A in Z. rouxii MRB1; (●) Residues making contact with the m6A base in Z. rouxii MRB1; (π) π-stacking Y205 in Z. rouxii MRB1 with G upstream of the m6A nucleotide; not conserved in human DC2; (π) π-stacking R296 in Z. rouxii MRB1 with the C on the 3′ side of the m6A nucleotide; (
) Residues making contact with bases other than m6A in Z. rouxii MRB1; (●) Residues making contact with the m6A base in Z. rouxii MRB1; (π) π-stacking Y205 in Z. rouxii MRB1 with G upstream of the m6A nucleotide; not conserved in human DC2; (π) π-stacking R296 in Z. rouxii MRB1 with the C on the 3′ side of the m6A nucleotide; (
 ) R1318 and R1322 in human DC2 form an increased positive surface charge than in human DC1. Amino acids involved in cage formation, RNA binding, hydrogen bond (H bond) formation are also indicated.
) R1318 and R1322 in human DC2 form an increased positive surface charge than in human DC1. Amino acids involved in cage formation, RNA binding, hydrogen bond (H bond) formation are also indicated.
The crystal structures show that the YTH domain does not have selective interactions with bases besides the 6-methyladenine. Instead, the YTH domain has selective recognition of phosphate and sugars in each YTH domain at the −2, −1, +1, and +2 positions (Box 1) [25, 28] (Figure 4).
Transcriptome-wide binding studies of overexpressed[19, 36] or endogenous YTH proteins [16], using crosslinking-immunoprecipitation (CLIP) methods demonstrated that most mammalian YTH proteins bind to the m6A motif in RNA. Indeed, analysis of the binding properties of endogenous DF proteins showed nearly identical and complete overlap in their binding sites throughout the transcriptome. The vast majority of DF-binding sites are located in the consensus site for m6A (Box 1) [37], and the distribution of DF protein-binding sites in the transcriptome – referred to as a metagene – precisely mimics the distribution of m6A on mRNA [16]. Overall, this supports the idea that no m6A site is uniquely bound by one DF protein compared to others. In contrast, an earlier study argued that DF1 and DF2 bind to distinct sites, with an overlap of only ~60% of their mapped sites [36]. This was unexpected since the DF proteins are nearly identical and show identical binding specificity towards m6A RNA [30]. The likely explanation for the discrepancy is how the RNA-binding sites were identified from the CLIP data. The earlier studies used a bioinformatics approach in which sites were called based on whether the CLIP data showed signals above a specific threshold [36]. Subthreshold sites were not called. This approach results in stochastic calling efficiencies for sites that are near the threshold. However, the more recent study focused on m6A sites rather than DF sites [16]. At each m6A site, read densities for each DF protein was compared, revealing that each m6A in the transcriptome has a highly similar ratio of DF1, DF2, and DF3 reads [16]. Thus, based on this analysis, it is clear that no m6A site is uniquely bound by one of the DF proteins compared to the others.
Unlike the DF proteins, DC1-binding sites show nearly complete overlap with m6A sites in nuclear RNAs, such as XIST and MALAT1. Thus, DC1 appears to be the nuclear reader, while the DF proteins bind predominantly to m6A in cytoplasmic mRNAs. iCLIP analysis of DF1, DF2, DF3, and DC1 shows binding to the 10 putative m6A residues in a C-m6A-G motif formed by METTL16 in the MAT2A mRNA [38] (Figure 5a). Notably, previous studies of the binding affinity of the YTH domain binding to m6A-containing RNAs did not include RNAs in the C-m6A-G motif. However, examination an m6A-containing RNA in which the m6A was present in a C-m6A-C motif [29, 30] revealed that the presence of C preceding the m6A did not reduce binding affinity. Because of this, the YTH domain likely recognizes m6A generated by both METTL3 and METTL16, despite different sequence contexts of these m6A residues. In addition, DC2 does not show clear binding to m6A based on CLIP studies [16]. Most m6A sites in mRNA sites lack a corresponding DC2 iCLIP signal [16]. Thus, DC2 does not exhibit clear m6A-binding properties. It is possible that DC2 binds m6A under unique circumstances or in certain cell types.
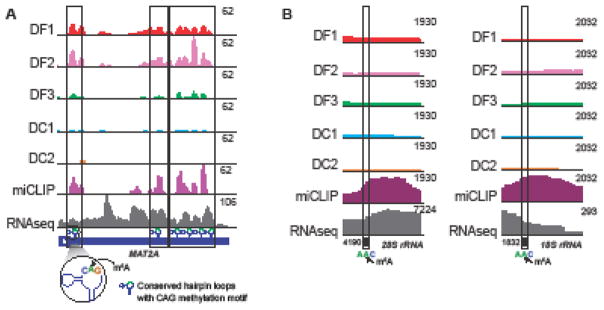
Figure 5. Recognition of m6A in human MAT2A mRNA and rRNAs.
(a) YTH domain proteins recognize m6A in CAG motif. 3′UTR of MAT2A mRNA contains m6A in an evolutionarily conserved CAG motif in U6-like hairpin loops. Shown here is a gene model of MAT2A 3′UTR (dark blue), m6A miCLIP tag profile (purple track) and iCLIP tag-based binding profiles of human DF1, DF2, DF3, DC1, and DC2 proteins. Dotted boxes mark the overlapping DF1, DF2, DF3 and DC1 peaks and the methylated regions on MAT2A 3′UTR. The green shaded portion on the hairpin loops marks the N6-methyladenosine containing CAG motif (zoomed-in view). (b) YTH proteins do not bind m6A on rRNAs. The highly prevalent m6A-containing 28S and 18S rRNAs each have one m6A modification in a AAC sequence motif. Shown is the location of m6A in 28S rRNA (left) and 18S rRNA (right), along with m6A peaks (miCLIP, purple), and the iCLIP binding profile of endogenous human YTH proteins. The absence of a peak in the YTH iCLIP reads show that none of the five YTH proteins bind at the m6A site on either 28S or 18S rRNA.
Do the YTH proteins bind m6A in 18S rRNA and 28S rRNA? These m6A are in an Am6A-C sequence [39]. Nevertheless, there is an absence of YTH iCLIP reads at m6A in these RNAs indicating that these residues do not bind YTH proteins (Figure 5b). The recently determined structure of human ribosomes show that each m6A residue in both 18S and 28S rRNA is buried within the subunit [40]. Thus, the lack of YTH binding may reflect the inaccessibility of these m6A residues.
Binding to other methylated forms of adenosine?
A highly prevalent modified form of adenosine is N6,2′-O-dimethyladenosine (m6Am) [41]. m6Am is found in 30–40% of all transcripts in vertebrate mRNA, adjacent to the m7G cap at the first encoded position of mRNAs [41]. Notably, m6Am markedly enhances mRNA stability and can be demethylated to 2′-O-methyladenosine (Am) by FTO [42]. FTO was initially thought to demethylate m6A [43], but recent studies show that FTO has essentially no activity towards m6A in vivo [42].
Could YTH domain proteins bind m6Am? DF1, DF2, and DF3 show specific recognition of the 2′-OH with a side chain asparagine (N230 in MRB1) through a hydrogen bond (Figure 4) [25, 27, 30], and in DC1 a distinct asparagine (N363 in human DC1) mediates this function. Thus, these YTH domains are unlikely to bind to m6Am.
Besides m6A and m6Am, adenosine can be methylated to N1-methyladenosine (m1A) and N6, N6-dimethyladenosine (m6,6A). A global mapping study of m1A proposed that mammalian mRNAs contain at least 7000 m1A residues [44, 45], but more recent analysis shows that just 7 cellular mRNAs contain m1A [46]. Nevertheless, the YTH domain is unlikely to mediate recognition of m1A because methylation of the N1 atom of the adenine ring would block hydrogen-bonding interactions that depend on the nonmethylated nitrogen. The YTH domain proteins also do not bind m6,6A, a modification in the 18S rRNA, based on CLIP data [16]. YTH proteins form interactions with the N6 hydrogen on m6A, which is not present in m6,6A (Figure 3). Taken together, the structural and CLIP studies suggest that the YTH domain does not bind other modified adenosines, such as m6Am, m1A or m6,6A.
A YTH domain that does not bind m6A
The YTH domain in the Schizosaccharomyces pombe protein Mmi1 does not show m6A-enhanced binding despite possessing an aromatic cage. The YTH domain binds a non-methylated RNA motif (UUAAAC), also known as the DSR (determinant of selective removal) motif [47]. However, DSR sequences with m6A do not show enhanced binding, and in some cases, display worse binding compared to the nonmethylated RNA. Structural studies of Mmi1 bound to the DSR RNA revealed that the RNA binds a different surface than the canonical RNA-binding surface seen in the other YTH proteins [47]. Thus, the aromatic cage is completely unoccupied when the DSR RNA is bound to the Mmi1 YTH domain. Thus, this YTH domain appears to bind nonmethylated RNAs despite the presence of an aromatic cage.
DF family: Redundant or different functions?
DF proteins are enriched in the cytoplasm [19, 36, 48–50], suggesting that they affect the fates of mRNA in the cytoplasm, such as mRNA stability and translation (Figure 6, Key Figure). A major question is whether YTHDF proteins have different or redundant functions. Initial studies of DF1 and DF2 showed that these proteins have highly distinct functions in promoting translation and mRNA instability, respectively [19, 36]. However, other studies have come to the opposite conclusion, and found that DF1, DF2, and DF3 function in a similar manner [51, 52]. The finding that these proteins have similar functions is consistent based on their sequence: DF1, DF2, and DF3 do not contain different modules that might confer unique properties to each protein. Additionally, lower organisms such as Drosophila express a single DF-like YTH protein, CG6422, rather than separate homologs for DF1, DF2, and DF3 [13], consistent with the idea that there is a single conserved function for these proteins.
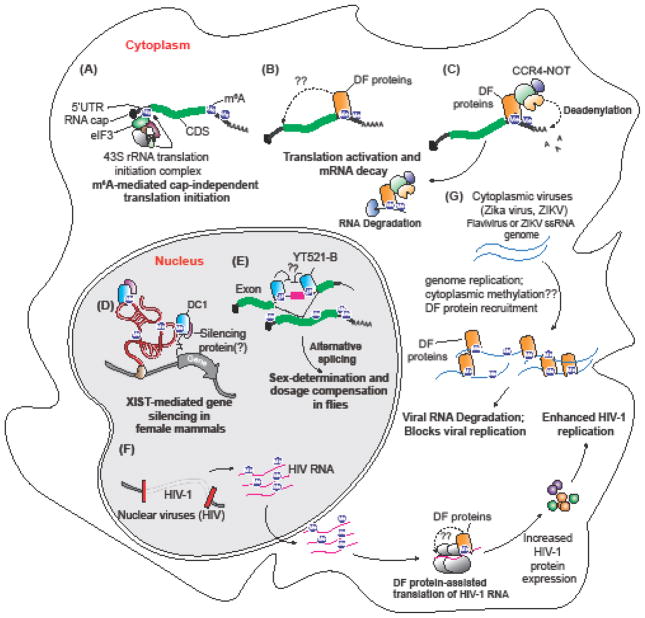
Figure 6, Key Figure. Multiple functions of YTH proteins.
(a) Cap-independent translation mediated by m6A and eIF3. A subset of transcripts contain m6As in their 5′UTR that can be recognized by eIF3. Binding of eIF3 recruits the 43S translation initiation complex leading to cap-independent translation. (b) YTHDF proteins regulate translation of transcripts by binding to m6A in the 3′UTR and recruiting eIF3. It is proposed that the mRNA circularizes to bring eIF3 in proximity to the cap in order to initiate translation. (c) YTHDF proteins promote mRNA decay. The C-terminal YTH domain binds m6A on transcripts and the N-terminal disordered region recruits CNOT1, a component of the CCR4-NOT complex. Following deadenylation by CCR4-NOT1 transcripts are decapped and degraded. (d) YTHDC1 is necessary for X-chromosome inactivation (XCI). XIST is a heavily methylated ncRNA bound by DC1. In the absence of m6A or DC1 X-chromosome inactivation is impaired. (e) YTHDC1 (YT-521B in Drosophila) binding to m6A is necessary for X chromosome dosage compensation and sex determination in flies. In female flies, the introns flanking the second exon of the sex-lethal (Sxl) transcript contain m6A and binding of DC1 promotes skipping of the second exon and production of full-length Sxl protein. (f) Nuclear replicating viruses, such as HIV, have highly conserved m6A sites that actively recruit DF proteins to promote translation of viral proteins. (G) Cytoplasmic replicating viruses, such as Zika virus, are methylated in the cytoplasm through an unknown METTL3-dependent mechanism and bound by the DF proteins. Binding of DF proteins to cytoplasmic virus transcripts confines the transcripts to lipid droplets in liver cells and inhibits viral replication.
mRNA stability
DF2 was originally implicated in mediating m6A-associated mRNA instability as knockdown of DF2 stabilized mRNAs that contained DF2-binding sites [19]. This appears to involve P-bodies as DF2 co-localization with P-bodies through its N-terminal P/Q/N-rich low complexity region [19]. However, other studies did not find DF2 in immunoprecipitates of GW182, a core component of P-bodies [52]. It is likely that the association DF2 with P-bodies is transient, which accounts for the difficulty in detecting DF2 in these bodies. Others have suggested that DF2 mediates mRNA degradation by first triggering mRNA deadenylation, followed by subsequent translocation to P-bodies [52]. Indeed, the N-terminus of DF2 was shown to bind to CNOT1, a component of the CCR4-NOT deadenylation complex, leading to deadenylation[52]. Notably, this study found that all DF proteins recruit CCR4-NOT and promote mRNA deadenylation [52]. This contrasts with earlier studies which had argued that DF1 has minimal effects on mRNA stability [36].
Although DF2 appears to contribute to mRNA stability, other m6A readers may also contribute to m6A-mediated mRNA instability. To address this, it will be critical to determine the extent to which m6A regulates mRNA stability in cells depleted of DF proteins.
Translational activation
Until recently, DF1 was the only DF protein expected to regulate translation. DF1 enhanced translational efficiency in mRNAs that are normally bound by DF1 [36] by direct binding of DF1 to eIF3. Since DF1-binding sites, like m6A, is primarily around the stop codon and 3′UTR, this model suggests that eIF3 is recruited near mRNA stop codons. Previous studies showed that eIF3-induced translation activation occurs by recruitment of eIF3 to 5′ UTRs, for example by viral sequences [53] and other translation-promoting elements [18, 54], but it remains unclear how this noncanonical stop codon recruitment of eIF3 would promote translation.
Recently, other DF proteins were shown to promote translation [51]. DF proteins that were tethered to a reporter RNA resulted in enhanced translation of the reporter [51]. This is partially supported in another study that showed that DF3 exhibits both translation enhancement and mRNA degradation upon binding its targets [48, 50]. Thus, DF3 was proposed to have a combination of the features of DF1 and DF2. As with DF1, a clear mechanism by which binding leads to translation is unclear and is needed in order to fully understand the role of DF1 and DF3 in translational enhancement.
A common theme in these more recent studies is that all three DF proteins share a similar, rather than distinct function. Each of these proteins has been shown to promote translation and each can induce RNA degradation. It will be important to definitively determine if this more recent model of redundant function explains the DF proteins or if the original concept of distinct translation and mRNA destabilization functions for DF1 and DF2 is accurate.
m6A: pro-viral or anti-viral?
m6A was found in Simian virus 40 (SV40), Rous sarcoma virus (RSV), Adenovirus-2 (Ad2), Herpes simplex virus type-1 (HSV-1), Avian sarcoma virus B77 (ASV), and Influenza virus (IAV) viral RNA since the 1970s [55–60]. However, the function of m6A in these viruses has been a mystery. In the case of HIV it appears that the virus is actively utilizing the host RNA methylation machinery for its replication. Loss of the m6A writing complex inhibits HIV replication [61, 62]. HIV recruits DF proteins to methylation sites and this enhances viral replication [51]. How DF proteins enhance viral replication remains unknown, but it seems to be inconsistent with the destabilizing function of the DF proteins on host cell-encoded mRNAs.
RNA viruses that replicate in the cytosol can also contain m6A. This is unexpected since METTL3 is classically thought to be nuclear [63], and methylation is thought to occur cotranscriptionally [64]. Nevertheless, various cytoplasmic RNA viruses exhibit m6A in their viral genome, including Zika virus, and other flaviviruses including dengue virus, West Nile virus, yellow fever virus, and hepatitis C virus [65, 66]. Unlike in HIV where m6A seems to promote viral replication, m6A in these viruses seem to suppress replication. In the case of hepatitis C virus (HCV), loss of m6A or the DF proteins enhanced the production of viral particles [66]. The mechanism of m6A-mediated suppression of viral replication does not appear to involve m6A effects on RNA stability or translation. Instead, HCV may require the formation of lipid droplets and that localization of the DF proteins to the droplets impairs the formation of viral particles [66].
YTHDC1
Unlike DF proteins, DC1-binding sites show nearly complete overlap with m6A sites in nuclear RNAs, such as XIST and MALAT1. Thus, DC1 influences nuclear processing and functions of nuclear-localized RNAs.
m6A-directed regulator of splicing
DC1 was originally characterized as a regulator of splicing by promoting exon inclusion [67]. The YTH domain was found to be essential for this effect [68]. Based on our current understanding that the YTH domain binds m6A, this finding indicates that the effect of DC1 on splicing is dependent on m6A [67]. Recent studies indicate that DC1 recruits SRSF3 to promote exon inclusion [68]. However, evidence for m6A in regulation of splicing is limited. The first in-depth analysis of altered splicing discovered that less than 100 exons containing an m6A site are alternatively spliced upon knockdown of METTL3 [6]. Further examination of m6A-induced splicing was analyzed in Mettl3−/− mouse embryonic stem cells, revealing that less than 0.5% of all exons exhibit alternative splicing and of those less than 100 contain an m6A site [7, 69].
While m6A may not globally affect splicing in mammalian cells, m6A regulates sex-specific gene expression by regulating splicing of sex-lethal (Sxl) in Drosophila [13–15]. Males express a Sxl transcript that includes an additional exon that contains a stop codon, thus preventing Sxl protein synthesis. In females, m6A in Sxl recruits YT521-B (the Drosophila DC1 ortholog), resulting in skipping of the exon and production of a transcript that encodes full-length Sxl protein. Loss of m6A results in impaired exon skipping in Sxl, which leads to a male-bias in the progeny. Overexpression of YT521-B is lethal to males [14]. Presumably this is because overexpression of YT521-B results in female-like splicing of Sxl, which inhibits translation of Male-Specific Lethal 2 (msl-2) leading to improper X-chromosome dosage compensation in males. Thus, m6A acts to directly regulate specific splicing events through YT521-B in Drosophila.
Regulating the epigenome through modulation of the epitranscriptome
DC1 mediates the action of the nuclear noncoding RNA XIST. XIST has an essential role in X-chromosome inactivation (XCI), a process that occurs in female mammalian cells. One X chromosome is randomly silenced in a process regulated by XIST, which coats the X chromosome and recruits chromatin-modifying factors that silence the chromosome [70]. XIST contains at least 76 m6A residues, and depletion of m6A from embryonic stem cells prevents XIST from inducing X chromosome gene silencing [16]. Of all the YTH proteins, DC1 preferentially binds to m6A sites in XIST, which is consistent with the nuclear localization of DC1. Loss of DC1 prevents XIST-mediated X chromosome gene silencing [16]. XCI can be restored in m6A-deficient cells by artificial tethering DC1 to XIST [16]. Thus, the primary role of m6A in XCI is to enable DC1 recruitment to XIST.
Concluding Remarks
Although the structure of the YTH domains is well understood, the functions of the YTH proteins remain unclear (see Outstanding Questions). In the case of DF proteins, a major question is whether these proteins are functionally redundant, or if they have different functions. Interpretation of experiments using knockdowns of DF proteins is hampered because DF2 is often more highly expressed than DF1 or DF3. Therefore, loss of DF1 or DF3 may not produce an effect similar to DF2 loss due to compensation. Deletion of all three DF proteins from cells followed by exogenous expression of one DF protein at a time may shed light on the ability of these proteins to perform redundant functions.
Outstanding Questions Box.
Box 1. Outstanding questions
Do the DF proteins have distinct or redundant functions? Independent studies report conflicting findings. These discrepancies need to be addressed with careful experimental design.
What is the role of post-translational modifications in controlling YTH proteins?
Does DC1 regulate chromatin modification globally through interacting with ncRNAs?
What is the function of DC2?
In addition, a clear m6A-dependent function for DC2 has yet to be described. DC2 is localized to the cytoplasm and can be induced by TNF-α [24, 71], raising the possibility that its function may be regulated in certain cell types through external stimuli. DC2 increased the translational efficiency of HIF1-α by unwinding its 5′UTR via the DC2 RNA helicase domain [72], but whether this is an m6A-dependent process remains unknown.
Relatively little information is also available on the regulation of YTH proteins. It is possible that DF proteins may be regulated differently based on the tissue type or in response to particular signals. Phosphoproteomic studies show numerous phosphorylation sites throughout the P/Q/N-rich region of the DF proteins and adjacent to the YTH domain [73]. Phosphorylation is known to prevent proteins from forming granules [74]. Therefore, it is possible that post-translational modifications regulate DF-mediated clustering interactions involved in liquid-liquid phase separation or granule formation. In addition to phosphorylation, DF proteins were found to be myristoylated in a proteomic study of myristoylated proteins [75]. However, it remains to be determined whether there are external or internal stimuli that lead to altered post-translational modifications. If so, how do these PTMs alter YTH protein interaction with other proteins or mRNA, or affect YTH localization? Currently, there are no known signaling pathways that modulate m6A. Therefore, any discovery in altered YTH regulation following such signals would advance our understanding of how m6A is regulated and what processes m6A regulates.
An important question is whether there are other mechanisms or readers by which m6A mediates its effects in cells, and the degree to which the actions of m6A can be ascribed to YTH proteins and eIF3. It will be important to examine whether m6A can still influence mRNA in cells deficient in YTH proteins.
An exciting yet unexplored area is the recent evidence that the epitranscriptome can regulate the epigenome based on studies of m6A in XIST [16]. The functions of m6A in XIST are mediated by its binding to DC1. If DC1 is indeed a general regulator of ncRNAs then m6A may have a general role in regulating chromatin marks.
m6A has a demonstrated importance in a number of diseases including viral infections and various cancers [51, 61, 62, 65, 66, 76–79]. This has garnered interest in developing small molecule inhibitors that target the m6A writer complex. However, another option that can be explored is pharmacologic targeting of the YTH proteins. The YTH domain forms a unique tryptophan cage necessary for the recognition and binding of m6As. Given the unique m6A is recognized by a well-defined binding pocket, this site may be suitable for small molecule inhibitors that would compete with m6A RNA and negate the effects YTH proteins. Therefore, better understanding of the m6A-binding proteins can reveal novel targets that can be used for therapeutic manipulation of the epitranscriptome.
Trends Box.
m6A is the most abundant RNA modification but its role in regulating RNA remains largely unknown. The YTH domain specifically recognizes m6A and is conserved from yeast to humans.
The first function assigned to a YTH protein was destabilization of mRNAs by YTHDF2. Since then YTH proteins have been found to increase translation (YTHDF1 and YTHDF3), and regulate splicing and chromatin modification (YTHDC1).
The YTHDF proteins have been found in stress granules and protein droplet structures implicating m6A in granule formation.
6A has been mapped in multiple viruses and binding of YTH proteins was shown to either help or inhibit viral replication. Nuclear replicating viruses are helped by YTH proteins while cytoplasmic replicating viruses are inhibited.
Acknowledgments
We thank members of the Jaffrey laboratory for their valuable comments and suggestions. Supported by NIH grants R01CA186702 (S.R.J), T32CA062948 (B.F.P.), and F32CA22104 (B.F.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perry RP, Kelley DE. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1(1):37–42. [Google Scholar]
- 2.Desrosiers R, et al. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clancy MJ, et al. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30(20):4509–18. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong S, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20(5):1278–88. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149(7):1635–46. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–6. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 7.Geula S, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347(6225):1002–6. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 8.Batista PJ, et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15(6):707–19. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16(2):191–8. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffrey SR, Kharas MG. Emerging links between m6A and misregulated mRNA methylation in cancer. Genome Med. 2017;9(1):2. doi: 10.1186/s13073-016-0395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu LP, et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017 doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fustin JM, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155(4):793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Kan L, et al. The m6A pathway facilitates sex determination in Drosophila. Nat Commun. 2017;8:15737. doi: 10.1038/ncomms15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haussmann IU, et al. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540(7632):301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 15.Lence T, et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540(7632):242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 16.Patil DP, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roost C, et al. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J Am Chem Soc. 2015;137(5):2107–15. doi: 10.1021/ja513080v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer KD, et al. 5′ UTR m(6)A Promotes Cap-Independent Translation. Cell. 2015;163(4):999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–20. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musa J, et al. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): a master regulator of mRNA translation involved in tumorigenesis. Oncogene. 2016;35(36):4675–88. doi: 10.1038/onc.2015.515. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y, et al. Cloning of a gene, YT521, for a novel RNA splicing-related protein induced by hypoxia/reoxygenation. Brain Res Mol Brain Res. 1998;53(1–2):33–40. doi: 10.1016/s0169-328x(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann AM, et al. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59(fyn) Mol Biol Cell. 1999;10(11):3909–26. doi: 10.1091/mbc.10.11.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoilov P, et al. YTH: a new domain in nuclear proteins. Trends Biochem Sci. 2002;27(10):495–7. doi: 10.1016/s0968-0004(02)02189-8. [DOI] [PubMed] [Google Scholar]
- 24.Jain D, et al. ketu mutant mice uncover an essential meiotic function for the ancient, putative RNA helicase YTHDC2. bioRxiv. 2017 doi: 10.1101/171827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo S, Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci U S A. 2014;111(38):13834–9. doi: 10.1073/pnas.1412742111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu T, et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24(12):1493–6. doi: 10.1038/cr.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, et al. Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res. 2014;24(12):1490–2. doi: 10.1038/cr.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theler D, et al. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res. 2014;42(22):13911–9. doi: 10.1093/nar/gku1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu C, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10(11):927–9. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
- 30.Xu C, et al. Structural Basis for the Discriminative Recognition of N6-Methyladenosine RNA by the Human YT521-B Homology Domain Family of Proteins. J Biol Chem. 2015;290(41):24902–13. doi: 10.1074/jbc.M115.680389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, et al. The dataset for protein-RNA binding affinity. Protein Sci. 2013;22(12):1808–11. doi: 10.1002/pro.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin S, Min J. Structure and function of the nucleosome-binding PWWP domain. Trends Biochem Sci. 2014;39(11):536–47. doi: 10.1016/j.tibs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Yap KL, Zhou MM. Structure and mechanisms of lysine methylation recognition by the chromodomain in gene transcription. Biochemistry. 2011;50(12):1966–80. doi: 10.1021/bi101885m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaver JE, Waters ML. Molecular Recognition of Lys and Arg Methylation. ACS Chem Biol. 2016;11(3):643–53. doi: 10.1021/acschembio.5b00996. [DOI] [PubMed] [Google Scholar]
- 35.Hughes RM, et al. Recognition of trimethyllysine by a chromodomain is not driven by the hydrophobic effect. Proc Natl Acad Sci U S A. 2007;104(27):11184–8. doi: 10.1073/pnas.0610850104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, et al. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161(6):1388–99. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linder B, et al. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12(8):767–72. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pendleton KE, et al. The U6 snRNA m6A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell. 2017;169(5):824–835. e14. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piekna-Przybylska D, et al. The 3D rRNA modification maps database: with interactive tools for ribosome analysis. Nucleic Acids Res. 2008;36(Database issue):D178–83. doi: 10.1093/nar/gkm855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khatter H, et al. Structure of the human 80S ribosome. Nature. 2015;520(7549):640–5. doi: 10.1038/nature14427. [DOI] [PubMed] [Google Scholar]
- 41.Wei C, et al. N6, O2′-dimethyladenosine a novel methylated ribonucleoside next to the 5′ terminal of animal cell and virus mRNAs. Nature. 1975;257(5523):251–3. doi: 10.1038/257251a0. [DOI] [PubMed] [Google Scholar]
- 42.Mauer J, et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature. 2017;541(7637):371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–7. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominissini D, et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530(7591):441–6. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, et al. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol. 2016;12(5):311–6. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 46.Safra M, et al. The m1A landscape on cytosolic and mitochondrial RNA at single base resolution. Nature. 2017 doi: 10.1038/nature24456. In press. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, et al. A novel RNA-binding mode of the YTH domain reveals the mechanism for recognition of determinant of selective removal by Mmi1. Nucleic Acids Res. 2016;44(2):969–82. doi: 10.1093/nar/gkv1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li A, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27(3):444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J, et al. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526(7574):591–4. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi H, et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy EM, et al. Posttranscriptional m(6)A Editing of HIV-1 mRNAs Enhances Viral Gene Expression. Elife. 2016;19(5):675–85. doi: 10.1016/j.chom.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du H, et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson RJ, et al. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11(2):113–27. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee AS, et al. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature. 2015;522(7554):111–4. doi: 10.1038/nature14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canaani D, et al. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979;6(8):2879–99. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kane SE, Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol. 1985;5(9):2298–306. doi: 10.1128/mcb.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommer S, et al. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976;3(3):749–65. doi: 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moss B, et al. 5′-Terminal and internal methylated nucleosides in herpes simplex virus type 1 mRNA. J Virol. 1977;23(2):234–9. doi: 10.1128/jvi.23.2.234-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimock K, Stoltzfus CM. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977;16(3):471–8. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- 60.Krug RM, et al. Influenza viral mRNA contains internal N6-methyladenosine and 5′-terminal 7-methylguanosine in cap structures. J Virol. 1976;20(1):45–53. doi: 10.1128/jvi.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tirumuru N, et al. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife. 2016:5. doi: 10.7554/eLife.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lichinchi G, et al. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2016;1:16011. doi: 10.1038/nmicrobiol.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bokar JA, et al. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3(11):1233–47. [PMC free article] [PubMed] [Google Scholar]
- 64.Slobodin B, et al. Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell. 2017;169(2):326–337. e12. doi: 10.1016/j.cell.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lichinchi G, et al. Dynamics of Human and Viral RNA Methylation during Zika Virus Infection. Cell Host Microbe. 2016;20(5):666–673. doi: 10.1016/j.chom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gokhale NS, et al. N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe. 2016;20(5):654–665. doi: 10.1016/j.chom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, et al. The YTH domain is a novel RNA binding domain. J Biol Chem. 2010;285(19):14701–10. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao W, et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 2016;61(4):507–19. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 69.Ke S, et al. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31(10):990–1006. doi: 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engreitz JM, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341(6147):1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanabe A, et al. Transcriptional machinery of TNF-alpha-inducible YTH domain containing 2 (YTHDC2) gene. Gene. 2014;535(1):24–32. doi: 10.1016/j.gene.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Tanabe A, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1alpha mRNA is translated. Cancer Lett. 2016;376(1):34–42. doi: 10.1016/j.canlet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 73.Hornbeck PV, et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43(Database issue):D512–20. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato M, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149(4):753–67. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thinon E, et al. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat Commun. 2014;5:4919. doi: 10.1038/ncomms5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dixit D, et al. Messenger RNA Methylation Regulates Glioblastoma Tumorigenesis. Cancer Cell. 2017;31(4):474–475. doi: 10.1016/j.ccell.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S, et al. m6A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell. 2017;31(4):591–606. e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C, et al. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7(40):64527–64542. doi: 10.18632/oncotarget.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwok CT, et al. Genetic alterations of m6A regulators predict poorer survival in acute myeloid leukemia. J Hematol Oncol. 2017;10(1):39. doi: 10.1186/s13045-017-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oates ME, et al. D(2)P(2): database of disordered protein predictions. Nucleic Acids Res. 2013;41(Database issue):D508–16. doi: 10.1093/nar/gks1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vilella AJ, et al. EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 2009;19(2):327–35. doi: 10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–5. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwartz S, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014;8(1):284–96. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534(7608):575–8. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 85.Wang P, et al. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016;63(2):306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]