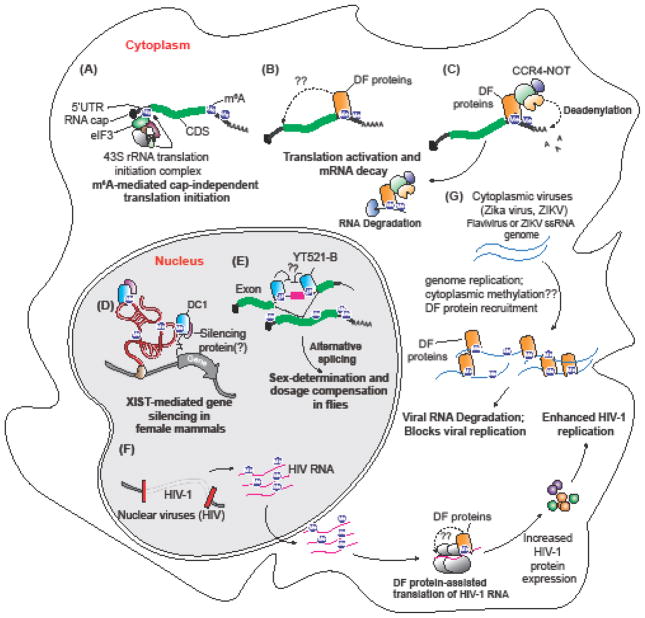
Figure 6, Key Figure. Multiple functions of YTH proteins.
(a) Cap-independent translation mediated by m6A and eIF3. A subset of transcripts contain m6As in their 5′UTR that can be recognized by eIF3. Binding of eIF3 recruits the 43S translation initiation complex leading to cap-independent translation. (b) YTHDF proteins regulate translation of transcripts by binding to m6A in the 3′UTR and recruiting eIF3. It is proposed that the mRNA circularizes to bring eIF3 in proximity to the cap in order to initiate translation. (c) YTHDF proteins promote mRNA decay. The C-terminal YTH domain binds m6A on transcripts and the N-terminal disordered region recruits CNOT1, a component of the CCR4-NOT complex. Following deadenylation by CCR4-NOT1 transcripts are decapped and degraded. (d) YTHDC1 is necessary for X-chromosome inactivation (XCI). XIST is a heavily methylated ncRNA bound by DC1. In the absence of m6A or DC1 X-chromosome inactivation is impaired. (e) YTHDC1 (YT-521B in Drosophila) binding to m6A is necessary for X chromosome dosage compensation and sex determination in flies. In female flies, the introns flanking the second exon of the sex-lethal (Sxl) transcript contain m6A and binding of DC1 promotes skipping of the second exon and production of full-length Sxl protein. (f) Nuclear replicating viruses, such as HIV, have highly conserved m6A sites that actively recruit DF proteins to promote translation of viral proteins. (G) Cytoplasmic replicating viruses, such as Zika virus, are methylated in the cytoplasm through an unknown METTL3-dependent mechanism and bound by the DF proteins. Binding of DF proteins to cytoplasmic virus transcripts confines the transcripts to lipid droplets in liver cells and inhibits viral replication.