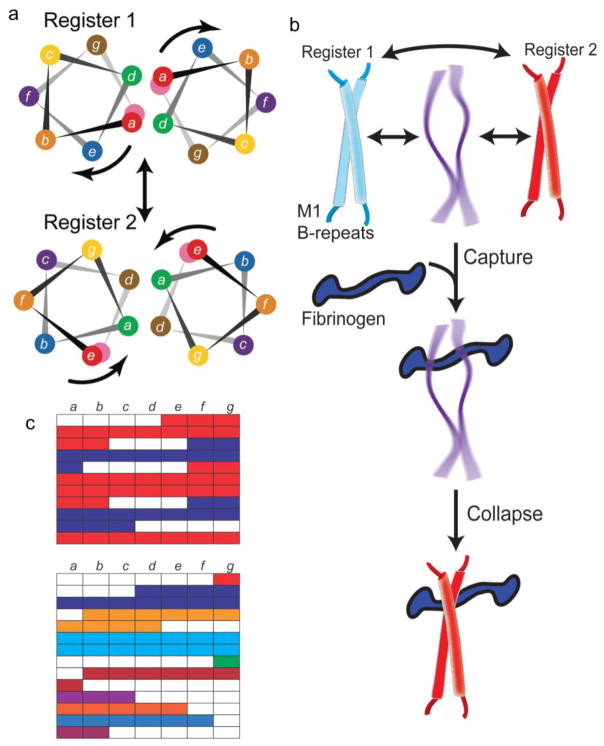
Figure 4. Protein Dynamics in the B-repeats.
a. Helical wheel representation of the M1 B-repeats in register 1 (top) and 2 (bottom). The color coding of residues is preserved in the two registers, and the arrows indicate the rotation of the helical face required to transition from one register to the other. Adapted from Stewart, C.M. et al. (2016) Proc Natl Acad Sci USA 113 (34), 9515–20 [16].
b. Interconversion between multiple conformations in the M1 B-repeats, with the central conformation representing a dissociated and natively unfolded state. The ‘capture-and-collapse’ model of Fg-binding is shown, with the natively unfolded state of the B-repeats capturing Fg, leading to collapse of the B-repeats into register 2.
c. Top, Heptad register predicted for the M1 B-repeats, with register 1 in blue and register 2 in red. Gaps are in white. Bottom, Heptad register predicted for the M5 B-repeats. Contiguous amino acids are colored similarly.