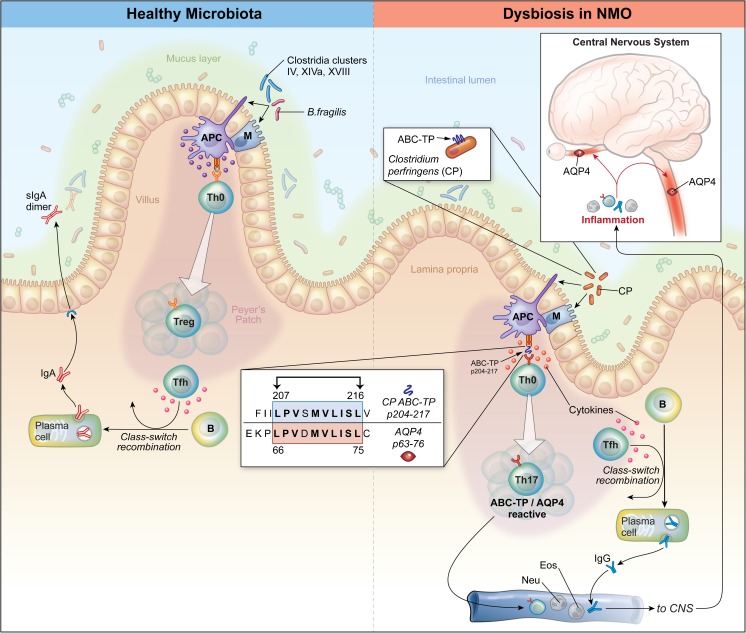
Fig. 4.
Model illustrating potential roles of Clostridium perfringens in neuromyelitis optica (NMO) pathogenesis. (Left) “Healthy microbiota”. Commensal bacteria, including Bacteroides fragilis and certain species within Clostridia clusters IV, XIVa, and XVIII, promote T-cell immune regulation [47, 49, 50]. Bacteria bind to M cells, which are concentrated in the terminal ileum and appendix in proximity to Peyer’s patches, a gut-associated lymphoid tissue (GALT), and are highly specialized to engulf microbial antigens and deliver them to antigen presenting cells (APCs) [55, 56], including dendritic cells (DC) and macrophages. APCs may also ingest bacterial antigens directly. The APCs, including regulatory CD103+ DC, produce anti-inflammatory cytokines (e.g., transforming growth factor-β) that promote expansion of antigen-specific regulatory T cells (Tregs). Those Tregs, along with T follicular helper cells (Tfh), a specialized subset of T cells that directs B-cell maturation, class-switch recombination, and differentiation into immunoglobulin-secreting plasma cells [18], promote production of bacteria-specific IgA [57, 58], which is the most abundant immunoglobulin subclass in the gastrointestinal tract. Individual IgA molecules enter gut epithelial cells and form IgA dimers, which are secreted (sIgA) into the intestinal lumen and mucus layer where they bind their specific bacterial targets. Bacteria-specific sIgA are known to alter microbiota composition and are thought to protect against inflammation and disease [57–59]. (Right) “NMO dysbiosis”. Overabundance of C. perfringens (CP) may elicit proinflammatory aquaporin-4 (AQP4)-specific T-cell and B-cell responses that contribute to development of NMO. CP binds to M cells or APC as described above. Processing of CP by APCs exposes a determinant of the ABC-TP (p204-217) that shares homology to AQP4 (p63-76), and when presented by APC, leads to activation and expansion of T cells that recognize either of these antigens (“molecular mimicry”) [31]. CP may expose products that promote secretion of the APC-derived proinflammatory Th17-polarizing cytokines (e.g., interleukin-6) that are increased in patients with NMO [31, 60, 61] leading to expansion of ABC-TP/AQP4-reactive T cells. Those Th17 cells, along with Tfh within GALT or in other secondary lymphoid tissues, promote AQP4-specific B cells to differentiate into plasma cells that secrete pathogenic AQP4-specific IgG1. In conjunction with other leukocytes (e.g., neutrophils and eosinophils), ABC-TP/AQP4-specific Th17 cells and AQP4-specific IgG target AQP4 in the central nervous system (CNS) causing inflammation of the optic nerves and spinal cord. Image courtesy of Xavier Studio