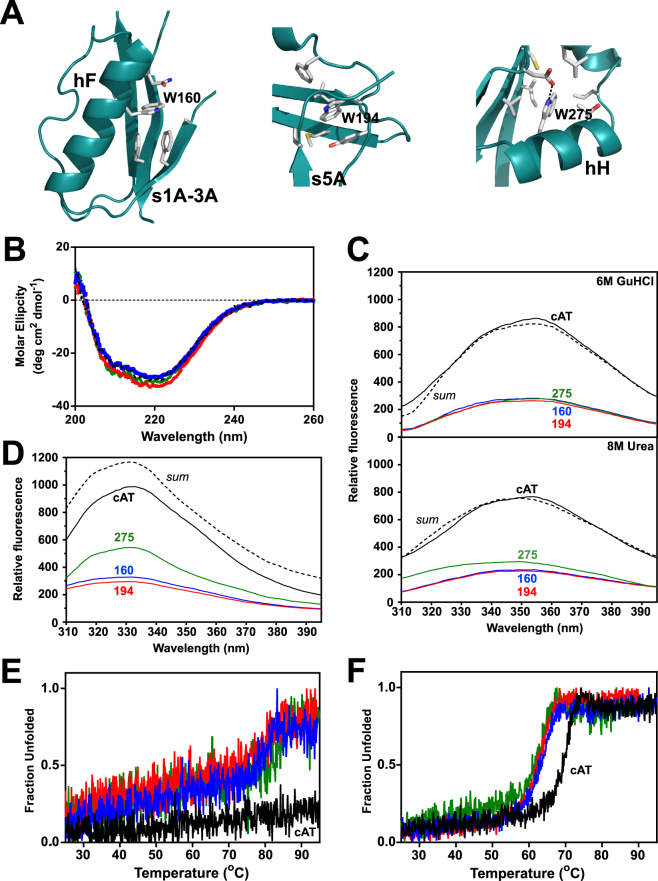
Figure 5.
Properties of single-tryptophan mutants of cAT. (A) Residues and structural elements proximate to tryptophans 160, 194 and 275 (α1-AT numbering) present in the structure of native conserpin (5CDX) are shown. (B) Far-UV spectra of cATW160 (blue) cATW194 (red) and cATW275 (green) are shown with respect to cAT (black dashes), at a concentration of 0.2 mg mL−1, using a path length of 0.1 cm, at 25 °C. (C) Fluorescence emission spectra for cAT (black), cATW160 (blue) cATW194 (red) and cATW275 (green), in the presence of 6 M of GdnHCl (upper panel) and 8 M urea (lower panel). The dashed spectrum is the summation of all tryptophan mutants. Sample fluorescence, with excitation at 295 nm and 5 nm slit widths, was buffer-corrected. (D) As in panel C, but in the absence of denaturant. (E) Single-tryptophan variants cATW160 (blue) cATW194 (red) and cATW275 (green) at 0.2 mg mL−1, heated from 25 °C to 95 °C at a rate of 1 °C min−1, with unfolding monitored by the change in CD signal at 222 nm. (F) As in panel E, performed in the presence of 2 M GdnHCl.