Abstract
The recently discovered adipokinetic hormone/corazonin-related peptide (ACP) is an insect neuropeptide structurally intermediate between corazonin (CRZ) and adipokinetic (AKH) hormones, which all demonstrate homology to the vertebrate gonadotropin-releasing hormone (GnRH). To date, the function of the ACP signaling system remains unclear. In the present study, we molecularly identified the complete open reading frame encoding the Aedes aegypti ACP receptor (ACPR), which spans nine exons and undergoes alternative splicing giving rise to three transcript variants. Only a single variant, AedaeACPR-I, yielding a deduced 577 residue protein, contains all seven transmembrane domains characteristic of rhodopsin-like G protein-coupled receptors. Functional deorphanization of AedaeACPR-I using a heterologous cell culture-based system revealed highly-selective and dose-dependent receptor activation by AedaeACP (EC50 = 10.25 nM). Analysis of the AedaeACPR-I and AedaeACP transcript levels in all post-embryonic developmental stages using quantitative RT-PCR identified enrichment of both transcripts after adult eclosion. Tissue-specific expression profiling in adult mosquitoes reveals expression of the AedaeACPR-I receptor transcript in the central nervous system, including significant enrichment within the abdominal ganglia. Further, the AedaeACP transcript is prominently detected within the brain and thoracic ganglia. Collectively, these results indicate a neuromodulator or neurotransmitter role for ACP and suggest this neuropeptide may function in regulation of post-ecdysis activities.
Introduction
Neuropeptides are structurally and functionally the most diverse class of signaling molecules that function in intercellular communication in multicellular organisms1. In insects, neuropeptides play a fundamental role in the regulation of various physiological processes including development, reproduction, osmoregulation, as well as behavior and feeding2. One of the first neuropeptides to be isolated and purified from insects was the adipokinetic hormone (AKH), which is produced by neurosecretory cells of the corpora cardiaca (CC), and is one of the most extensively studied family of neuropeptides3. Insect AKH neuropeptides have been functionally well characterized to stimulate the release of energy-rich compounds including diacylglycerols, trehalose, and in some cases proline, into the haemolymph to fuel the activity of the insect4. In addition to these catabolic actions, AKH peptides have been shown to stimulate heart beat and inhibit the synthesis of haemolymph and tissue proteins5. The AKH receptors (AKHR), first isolated from Drosophila melanogaster and Bombyx mori, are G-protein coupled receptors (GPCR)6,7. Both AKH and AKHR demonstrate homology with the vertebrate gonadotropin releasing hormone (GnRH) and its receptor (GnRHR), respectively6,8.
Another insect neuropeptide family that is structurally similar to the vertebrate GnRH signaling system are the corazonin peptides. Corazonin (CRZ) is an insect undeca-neuropeptide first discovered in the cockroach, Periplaneta americana, due to its significant cardioexcitatory activity on the isolated cockroach heart9. A similar cardioexcitatory role has been established in other insects, including the kissing bug, Rhodnius prolixus10; however, this cardiostimulatory function is not universal since corazonin lacks cardiomyotropic activity in adult stage Anopheles gambiae mosquitoes11. Despite the CRZ sequence remaining largely conserved across insect species (primarily pQTFQYSRGWTNamide), to date no universal function has been described for this neuropeptide12. Nonetheless, multiple physiological roles have been described for CRZ, including the induction of melanization in swarming populations of Locusta migratoria and Schistocerca gregaria, initiation of ecdysis in Manduca sexta, the reduction in silk spinning rates in B. mori, and in social insects, CRZ has been proposed as a central regulator of behaviour and caste identity13–16.
Over the last decade, a third signaling system evolutionarily- and structurally-related to the AKHRs and CRZRs and their neuropeptides was identified and named adipokinetic hormone/corazonin-related peptide (ACP)17. ACP and its receptor (ACPR), like AKH/AKHR and CRZ/CRZR, also demonstrate homology to the vertebrate GnRH/GnRHR system. Upon analysis of this structural intermediate it became evident that ACP and its receptor ACPR were in fact already described in a number of insects, however, were at the time characterized as AKHs and AKHRs18–24. Unlike AKH, but similar to CRZ, the ACP amino acid sequence beginning from the N-terminus (pQVTFSRDW) demonstrates conservation across most arthropods17. Characterization of the ACP signaling system in R. prolixus, Tribolium castaneum, and A. gambiae revealed that the AKH, CRZ, and ACP receptors were only activated by their corresponding ligand, and thus are suggested to be independent signaling systems17,25. However, in vitro studies characterizing B. mori AKHR and ACPR have shown that high concentrations of ACP can activate the AKH receptor, and vice versa22,23. In both R. prolixus and A. gambiae, it was also shown that CRZ cannot activate either ACPR or AKHR and furthermore, neither AKH nor ACP can activate CRZR, which indicates that the AKH/AKHR and ACP/ACPR signaling systems are more closely related to one another17,25. To date, no functions have been assigned to the ACP signaling system, but expression profiles of ACP and ACPR in R. prolixus, and T. castaneum, show that both peptide and receptor are primarily expressed in the nervous system and, to a lesser extent, in the reproductive tissue of R. prolixus17,25. Furthermore, ACP transcript was detected in the head and thorax of adult Aedes aegypti with a similar transcript distribution observed in 4th instar larvae, pupae, and adult A. gambiae20,21. Developmental expression profiles have revealed high expression of both ACP and its receptor before and after hatching of eggs in T. castaneum and after ecdysis in R. prolixus, and thus a role in early larval development and post-ecdysis, respectively, has been proposed17,25. Additionally, assays testing the potential for cardio-excitatory or lipid mobilization roles of ACP in R. prolixus yielded negative results, which indicates that ACP, AKH, and CRZ are indeed independent signaling systems with distinct functions10. Further investigations are clearly necessary in order to assign a physiological role for the ACP/ACPR neuropeptide system in insects.
Aedes aegypti mosquitoes are principal vectors for a variety of pathogens including dengue fever, yellow fever, chikungunya, and Zika viruses, all of which have a significant impact on human morbidity and mortality26. A thorough understanding of mosquito biology is required to devise novel methods to reduce and prevent mosquito-borne diseases. In an attempt to advance our understanding of the ACP signaling system in A. aegypti, we have identified and functionally deorphanized the A. aegypti ACPR (AedaeACPR). Furthermore, to begin identification of prospective target tissues and physiological roles, we have determined the post-embryonic developmental expression profile and the spatial expression pattern in the adult mosquito of the transcripts encoding the ACP precursor peptide as well as a functional ACP receptor in A. aegypti.
Materials and Methods
Animals
Aedes aegypti (Liverpool strain) eggs were hatched in plastic containers half-filled with deionized water at an initial density of approximately 100 larvae/litre of water. Larvae were fed a 2% brewers yeast, 2% liver powder solution daily, and adults were provided with a 10% sucrose solution through a microcentrifuge tube fitted with a cotton ball wick allowing feeding ad libitum. Larvae and pupae were maintained in an incubator at 26 °C on a 12:12 hour light: dark cycle. Colony upkeep involved adult females being fed sheep’s blood in Alsever’s solution weekly (Cedarlane Laboratories Ltd., Burlington, ON) using an artificial feeding system described previously27. All experiments on adults were performed on either one or four-day old male and female mosquitoes that were sucrose-fed only and had been isolated during the pupal stage and transferred en masse into small glass microchambers.
Isolation and cloning of cDNA encoding A. aegypti ACPR
Gene specific forward and reverse primers were designed using Primer 3 in Geneious Software (Biomatters Ltd, Auckland, New Zealand) based on a predicted incomplete A. aegypti ACPR sequence (XM_001653870) described previously17 to amplify a 975 bp partial fragment using Q5 High Fidelity DNA Polymerase (New England Biolabs, Whitby, On) and whole adult female A. aegypti cDNA as template. The PCR product was purified, A-tailed, cloned into pGEM-T vector (Promega, Madison, WI, USA) and nucleotide sequence was confirmed by Sanger sequencing (Center for Applied Genomics, Hospital for Sick Children, Toronto, ON). After successful validation of the cloned partial sequence, primers were designed (as mentioned above) to perform 5′ and 3′ rapid amplification of cDNA ends (RACE)-PCR utilizing the Clontech SMARTer 5′/3′ RACE Kit (Takara BIO USA Inc, CA, USA). To facilitate cloning of amplicons, the linker sequence GATTACGCCAAGCTT, which overlaps with the pRACE vector provided in the kit, was added to the 5′ ends of the gene specific primers (Table 1). First-strand cDNA synthesis was prepared using 1μg total RNA from adult female head using the 3′ CDS primer (provided in the kit) and a gene-specific reverse primer to generate template cDNA for 3′ and 5′ RACE, respectively. Initial attempts at 5′RACE using the 5′CDS primer (provided in the RACE kit) for first-strand cDNA synthesis and subsequent PCR with the SeqAmp DNA Polymerase (Takara BIO USA Inc, CA, USA) was not successful for this target. Thus, the protocol was modified to generate first-strand cDNA using a gene-specific reverse primer (AedaeACPR-R1, Table 1) and subsequent nested RACE-PCR reactions utilized Q5 High Fidelity DNA Polymerase in lieu of SeqAmp DNA Polymerase. Nested PCR reactions utilized gene specific forward (3′ RACE) and reverse (5′ RACE) primers and a universal primer mix (UPM) to amplify the complete cDNA encoding A. aegypti ACPR. Optimal PCR cycling parameters for subsequent amplification of ACPR were determined empirically. Specifically, for 3′ RACE this included an initial denaturation at 94 °C for 1 min, followed by 40 cycles of 30 s at 94 °C, 30 s at 68 °C, and 3 min at 72 °C to amplify PCR products using SeqAmp DNA Polymerase. For 5′ RACE, the Q5 High Fidelity DNA Polymerase was utilized with the following cycling parameters, 30 s at 98 °C, followed by 30 cycles of 5 s at 98 °C, 15 s at 65–68 °C, 1 min 10 s at 72 °C, with a final extension step of 2 min at 72 °C. Following two rounds of PCR using nested gene-specific primers, amplicons were gel extracted and cloned into the linearized pRACE vector and miniprep samples were then sent for sequencing. Finally, primers were designed at the 5′ and 3′ ends of the complete cDNA sequence (including UTRs) and at the start and stop codons of the sequence (region including only the open reading frame, excluding UTRs), and were used for subsequent PCR amplification of the receptor with Q5 High Fidelity DNA polymerase to confirm base pair accuracy.
Table 1.
Primer information for oligonucleotides used for initial amplification of partial cDNA, 5′ and 3′ RACE and RT-qPCR analysis to determine temporal and spatial transcript expression.
| Oligo Name | Sequence (5′- > 3′) | Function |
|---|---|---|
| AedaeACPR-F1a | GGTCACACCGAAACGACAGTGG | Amplification and cloning of partial AedaeACPR |
| AedaeACPR-R1a | TGGACCTCCTCTGGGCTGC | Amplification and cloning of partial AedaeACPR and 1st strand cDNA synthesis for 5′ RACE |
| AedaeACPR-R2 | GACCGATTGGAGATTTCACAC | 5′RACE |
| AedaeACPR-R3 | CAGAACAGGTTGTAAGCCGTCT | 5′RACE |
| AedaeACPR-R4 | TCCACCGAGCATTATTTTGC | 5′RACE |
| AedaeACPR-R5 | TCCAGTGGGATCATGATGAAG | 5′RACE |
| AedaeACPR-LR5 | GATTACGCCAAGCTTCCTCCAGTGGGATCATGATGAAG | 5′RACE and cloning of 5′ RACE amplicon |
| AedaeACPR-LF1 | GATTACGCCAAGCTTGGTCACACCGAAACGACAGTGG | 3′ RACE |
| AedaeACPR-LF2 | GATTACGCCAAGCTTGTTGGATCGGTGCTTTGCTGTGAT | 3′ RACE |
| AedaeACPR-LF3 | GATTACGCCAAGCTT GGCTTACAACCTGTTCTGCGTGG | 3′ RACE and cloning of 3′ RACE amplicon |
| AedaeACPR-ORFkozak-F | GCCACCATGTATCTTTTCGGCAGGATTGCG | ORF cloning for functional receptor assay |
| AedaeACPR-ORF-R | TGATTTATCATCGCCAGCCACC | ORF cloning for functional receptor assay |
| AedaeACPR-N4721-F | AGGAATGGCAGCACCG | 5′-phosphorylated primers for generation of ACPR-I-N472I variant |
| AedaeACPR-N4721-R | GGCGAAGATCCCGTTG | 5′-phosphorylated primers for generation of ACPR-I-N472I variant |
| AedaeACPR-qPCR-F | GGGATGCGACTTCGTTGTA | qPCR amplification of AedaeACPR-I |
| AedaeACPR-qPCR-R | TCGCGGTCAAACATGTACC | qPCR amplification of AedaeACPR-I |
| AedaeACP-qPCR-Fb | ATGTGTTCTCTAAGGCGAAATAGC | qPCR amplification of AedaeACP |
| AedaeACP-qPCR-Rb | TTACAGGTGCCCATTCGAA | qPCR amplification of AedaeACP |
aPrimers based on partial ACPR mRNA sequence (Accession number: XM_001653870.216) identified through homology based in silico analysis of the A. aegypti genome.
bPrimers based on ACP mRNA sequence (Accession number: FM391984.119).
Gene Structure and Phylogenetic Analyses
Mapping of exon-intron boundaries of A. aegypti ACPR gene was determined using the cloned complete cDNA sequence as a query against the A. aegypti genome scaffolds database available locally on a lab computer running Geneious Pro Bioinformatics Software (Biomatters Ltd, Auckland, New Zealand). Positions of introns and exons were further confirmed using the BDGP splice site prediction server using the standardized data set of D. melanogaster genes28. Membrane topology of ACPR-I, II, and III were predicted using the Constrained Consensus TOPology prediction server (CCTOP)29. The deduced AedaeACPR-I, II and III protein sequences were aligned to the human gonadotropin-releasing hormone receptor 1 along with ACP, AKH, and CRZR receptors from other species (see Table S1) using ClustalW in MEGA 6.0630. Relationships between the various receptor sequences were determined through neighbour-joining31 and maximum-likelihood phylogenetic analysis methods32. Bootstrap values are based on 1000 replicates.
Preparation of mammalian expression constructs
Amplicons encoding just the open reading frame (start ATG to stop codon) were used as template for re-amplification using a modified forward primer possessing the consensus Kozak translation initiation sequence33,34 at the 5′ end of the start codon. The resulting product was cloned into pGEM-T Easy vector and then subcloned into the mammalian expression vector, pcDNA 3.1+ (Life Technologies, Burlington, ON). Construct directionality was confirmed by Sanger sequencing and plasmid DNA was purified from overnight bacterial cultures using a PureLink MidiPrep Kit (Invitrogen, Burlington, ON) and subsequently used for transfection of mammalian cells for the receptor functional assay. Analysis of multiple independent sequences obtained from RACE-PCR revealed a number of single nucleotide polymorphisms (SNPs) that localized to various sites along the full cDNA sequence. Upon further analysis, it was determined that only a single SNP at nucleotide position 1924 within the open reading frame resulted in an amino acid change. To determine whether this difference in the resulting residue, in comparison to the A. aegypti genome database, confers any difference to the functional activity of the receptor, site directed mutagenesis was performed. Specifically, 5′ phosphorylated primers were designed (Table 1) with the forward primer possessing an adenine (position 1924) consistent with the A. aegypti genome sequence whereas our consensus sequence contained a thymine in this nucleotide position. Using these modified primers, asymmetric PCR was performed using a pGEM-T Easy plasmid construct as template to replace the Ile472 in the cloned receptor with an Asn472 matching the A. aegypti genome. Mutation of the coding sequence was verified by sequencing and sub-cloned into the mammalian expression plasmid, pcDNA3.1+ (as described above).
Cell culture, transfections, and bioluminescence assay
Functional activation of AedaeACPR-I was assayed using a previously established cell culture system involving a recombinant Chinese hamster ovary (CHO)-K1 cell line stably expressing aequorin35. Cells were maintained in Dulbecco’s modified eagles medium: nutrient F12 (DMEM:F12; 1:1) media containing 200ug/mL geneticin, 10% heat-inactivated fetal bovine serum (FBS; Wisent, St. Bruno, QC) and grown to approximately 90% confluency, and were transiently transfected with pcDNA3.1+ expression vector possessing AedaeACPR-I using Lipofectamine LTX and Plus Reagent transfection system (Invitrogen, Burlington, ON) following a 3:1:1 transfection reagent (μL): Plus reagent (μL): plasmid DNA (μg) ratio. At 48 hours post-transfection, cells were detached from the culture flasks using Dulbecco’s phosphate buffered saline (DPBS; Wisent Corporation, St. Bruno, QC) containing 5 mM EDTA and resuspended in BSA medium (DMEM-F12 media containing 0.1% bovine serum albumin, 1X antimycotic-antibiotic) to a concentration of 106–107 cells/mL. Coelenterazine h (Promega, Madison, WI, USA) was added to the cells to a final concentration of 5 μM, and incubated for 3 hours in the dark at room temperature on a stirrer set at 200 rpm. The cell suspension was then diluted 10-fold and incubated at room temperature for an additional hour. Serial dilutions of peptide ligands (Table 1) were prepared in BSA medium (10−5 to 10−12 M), and loaded in quadruplicates into 96-well luminescence plates (Greiner Bio-One, Germany). All peptides were commercially synthesized (Genscript, Piscataway, NJ) at a purity >90% and were prepared in dimethyl sulfoxide at a stock concentration of 1 mM. Cells were loaded into each well with an automatic injector unit and luminescence was measured for 20 seconds using a Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). BSA medium alone was utilized as a negative control and 5 × 10−5 M ATP was used as a positive control, which acts on endogenously expressed purinoceptors36,37. EC50 values were calculated in GraphPad Prism 7.02 (GraphPad Software, San Diego, USA) from dose-dependent curves from four independent transfections.
Tissue dissections, RNA extraction, and cDNA synthesis
Lightly CO2-immobilized four-day old adult male (n = 30) and female (n = 20) A. aegypti were submerged in DPBS, and the following body segments and/or tissues were dissected and isolated: head, midgut, Malpighian tubules, hindgut, ovaries, testes, accessory reproductive tissues, and carcass (remaining fat body, musculature, and cuticle). Tissues were lysed in RNA lysis buffer containing 1% 2-mercaptoethanol. Whole adult RNA was obtained from submerging several males and females in RNA lysis buffer containing 1% 2-mercaptoethanol and using a sterile plastic pestle to disrupt the tissue. To measure the developmental expression profile for AedaeACPR, first to fourth instar larvae, pupae, as well as one- and four-day old adult mosquitoes were collected and submerged in RNA lysis buffer and flash frozen in liquid nitrogen. Total RNA was isolated from whole animal and individual adult tissues samples mentioned above using the PureLinkTM RNA mini kit following manufacturer protocol with an on-column DNase treatment to remove genomic DNA (Invitrogen, Burlington, ON). Purified total RNA samples were quantified with a Take3 micro-volume plate and measured on a Synergy Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). To assess ACP and ACPR transcript levels, cDNA was synthesized from 20 ng total RNA using the iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad, Mississauga, ON) following manufacturers protocol, including a ten-fold dilution of cDNA following synthesis.
Quantitative PCR
ACP and ACPR transcript abundance was quantified on a StepOnePlus™ Real Time PCR system (Applied Biosystems, Carlsband, CA) using PowerUP™ SYBR® Green Master Mix (Applied Biosystems, Carlsband, CA). Cycling conditions were as follows: 1) UDG activation 50 °C for 2 min, 2) 95 °C for 2 min, and 3) 40 cycles of (i) 95 °C for 15 seconds and (ii) 60 °C for 1 minute. Gene-specific primers designed over different exons were used to amplify ACPR, with the forward primer designed over exon 5 (nucleotides 1458–1476) to ensure specificity for ACPR-I, and the reverse primer over exon 6 (nucleotides 1585–1603). Gene specific primers amplifying AedaeACP were designed over multiple exons (Table 1; forward: nucleotides 89–112, reverse: nucleotides 403–421) based on a previously published mRNA sequence (Genbank Accession Number: FN391984)20. Relative expression levels were determined using the ΔΔCT method and were normalized to the geometric mean of rp49, rpL8, and rps18 reference genes, which were previously characterized and determined as optimal endogenous controls38. The AedaeACPR spatial expression profile was determined using 7–9 biological replicates, all of which included three technical replicates per reaction and a no-template negative control. The AedaeACPR developmental expression is an average of 3–5 biological replicates that each included duplicate technical replicates for each target gene and a no-template negative control. The AedaeACP spatial expression profile consisted of 3–4 biological replicates and the developmental expression is an average of 3–5 biological replicates. Specificity of primers for target mRNA were assessed by conducting no reverse-transcriptase controls, analysis of dissociation curves, and Sanger sequencing of amplicons. Data were analyzed using a one-way ANOVA with Dunnett’s multiple comparisons test where p < 0.05 was considered significant.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Results and Discussion
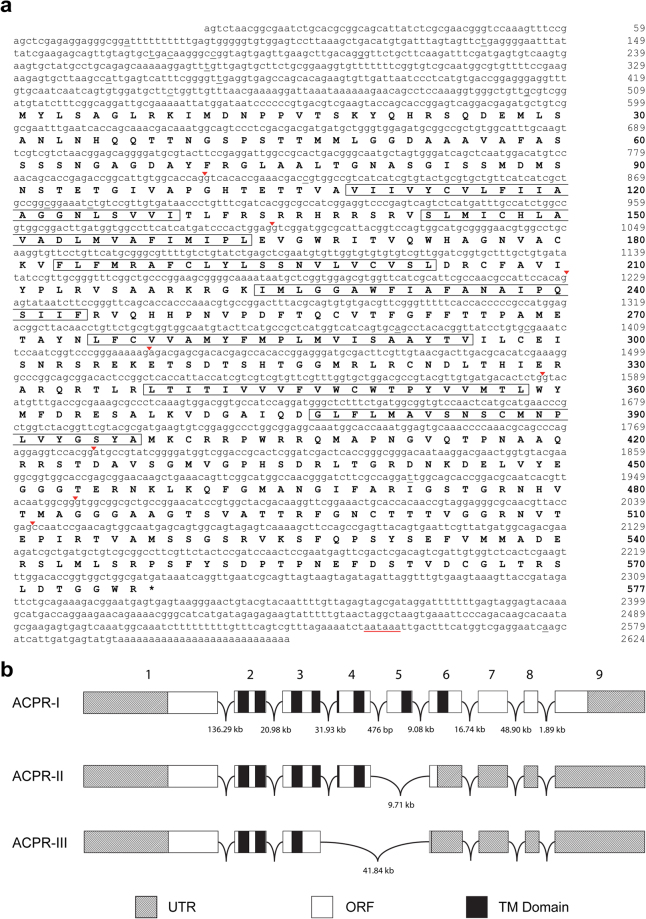
We have identified the complete cDNA sequence encoding the A. aegypti adipokinetic hormone/corazonin-related peptide (ACP) receptor (Fig. 1a). Following initial cloning and sequence analysis, three transcript variants were identified, AedaeACPR-I, ACPR-II, and ACPR-III (Fig. 1b). AedaeACPR-I is 2596 bp (GenBank Accession number: MF461644), which includes a 1734 bp open reading frame (ORF) encoding a 577 residue receptor (Fig. 1a). The cloned 5′ untranslated region (UTR) is 509 bp in length and the 3′UTR is 346 bp and contains a predicted polyadenylation sequence (nucleotide position 2547–2552). AedaeACPRs II and III are transcript variants of 2442 bp (GenBank: MF461645) and 2240 bp (GenBank: MF461646) in length, which yield deduced proteins comprised of 328 and 243 amino acids, respectively. Only AedaeACPR-I has the seven expected hydrophobic transmembrane (TM) domains characteristic of GPCRs, whereas AedaeACPR-II has only five TM domains and AedaeACPR-III has only three TM domains (see Fig. S1). Similarly, previous studies in R. prolixus25 and A. gambiae17 revealed multiple receptor variants, whereas only a single variant was identified in T. castaneum17. Specifically, in R. prolixus, three ACPR variants have been identified, two of which contain the typical seven TM domains (RhoprACPR-B and RhoprACPR-C), and one which contains five TM domains25. Additionally, two ACPR isoforms have been identified in A. gambiae, AnogaACPR-A and AnogaACPR-B, both of which result in receptors possessing seven TM domains17.
Figure 1.
Aedes aegypti adipokinetic hormone/corazonin-related peptide receptor variant I (ACPR-I) cDNA, amino acid sequence, and predicted gene structure. (a) Lower case letters denote cDNA, and uppercase letters represent amino acid residues with positions denoted by the numbers on the right of the sequences with bolded numbers indicating amino acid positions. Exon-exon boundaries within the cDNA are denoted by inverted red arrowheads. Underlined nucleotides indicate single nucleotide polymorphisms that differed from the A. aegypti genome. A putative polyadenylation signal is underlined in red in the 3′UTR. The predicted hydrophobic alpha-helices that form the transmembrane domains are outlined by black rectangular boxes. (b) The splicing pattern of the AedaeACPR gene based on BLAST analyses of cloned cDNA and splice site prediction analysis. Alternative splicing gives rise to three receptor mRNA variants where ACPR-I possesses all exons, ACPR-II lacks exon 5, and ACPR-III lacks both exons 4 and 5. Boxes representing exons are drawn to scale whereas intervening introns are not drawn to scale.
The gene structure was modeled by comparing the cloned cDNA sequence to the A. aegypti genome database using Geneious Bioinformatics software (Biomatters Ltd., Auckland, New Zealand). Analysis of the A. aegypti ACPR-I receptor shows that it maps over nine exons that are 810 bp, 191 bp, 228 bp, 202 bp, 154 bp, 197 bp, 177 bp, 83 bp, and 549 bp long, respectively. Alternative splicing of the A. aegypti ACPR gene results in the absence of either exon 5 only or absence of both exons 4 and 5, which yields AedaeACPR-II and AedaeACPR-III, respectively, both resulting in-frame translation shifts and premature stop codons, and consequently, truncated ORFs (Fig. 1b).
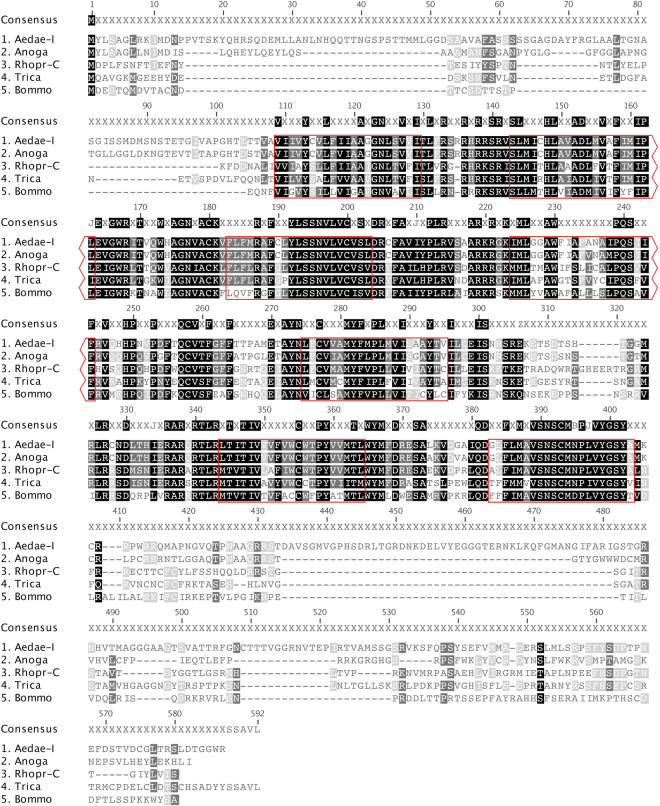
Alignment of A. aegypti ACPR-I, with selected receptors from A. gambiae, T. castaneum, R. prolixus, and B. mori, reveals conservation of the ACP receptor across insect species (Fig. 2). Specifically, AedaeACPR-I shares 59.4% sequence identity with the A. gambiae ACP receptor, 42.4% identity with the R. prolixus ACPR-C, 41.5% identity with the T. castaneum ACPR and 33.8% identity with the B. mori ACP receptor. Overall, there is a high degree of conservation over the seven predicted TM domains, particularly over TM regions one, two, three, five and seven. Strong sequence identity is also observed in the first and second intracellular loops, as well as the first extracellular loop. All of the receptor sequences, except for BommoACPR, which harbors an Asp in place of Asn, possess the conserved NPXXY motif in the seventh TM domain characteristic of rhodopsin-like (family A) GPCRs39,40. Another conserved motif found in rhodopsin-like GPCRs is the E/DRY motif adjacent to the second intracellular loop40. The Arg of the E/DRY motif and a negatively charged residue on TMVI of the GPCR undergo ionic interactions, known as the ionic lock, which stabilizes the inactive state of the receptor41. In particular, the ACP receptors possess a DRF motif in the silkworm B. mori, and DRC motifs are found in the mosquitoes A. gambiae and A. aegypti, in place of the characteristic DRY motif found in hemipteran R. prolixus and coleopteran T. castaneum, which have more conserved features of rhodopsin-like GPCRs40,42.
Figure 2.
Sequence alignment of select insect adipokinetic hormone/corazonin-related peptide receptors (ACPR). Aligned amino acid sequences of ACPRs from A. aegypti (Variant I, GenBank: MF461644) R. prolixus (GenBank: AKO62858), B. mori (GenBank: NP_001127726), T. castaneum (GenBank: ABX52400), and A. gambiae (GenBank: ABX52399). Residues outlined in red indicate predicted transmembrane domains based on the A. aegypti ACPR sequence. Highlighting of residues indicates % identity with black denoting 100% sequence identity, dark grey denotes 80–100% identity, and light grey represents amino acid positions with 60–80% sequence identity.
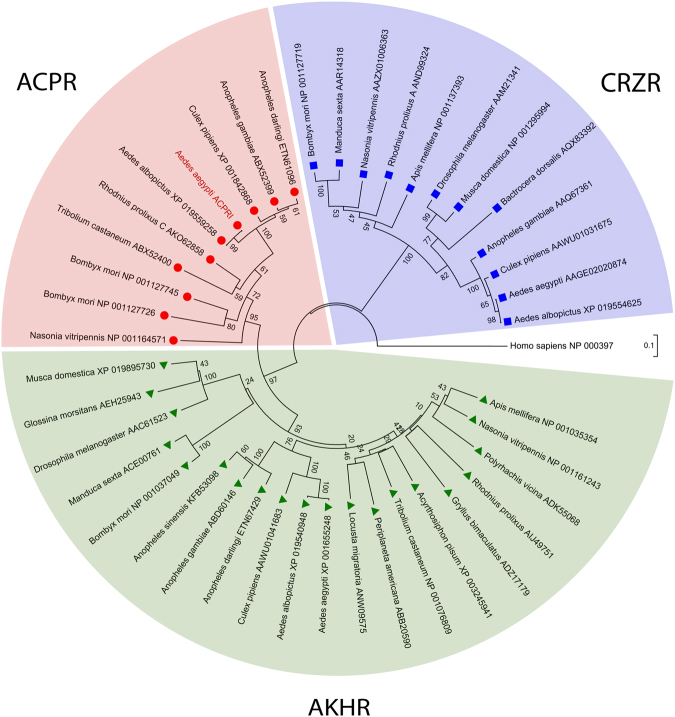
Phylogenetic analysis using the neighbor-joining and maximum-likelihood methods (not shown) yielded trees with highly similar and well supported topologies (Fig. 3). All the ACP receptors analyzed are positioned within a single clade that is a sister group to the clade comprised of the AKH receptors. Together, the AKH and ACP receptor clades form a monophyletic group which is a sister group to the clade comprised of CRZ receptors. The AedaeACPR-I identified herein clusters closely with the other insect ACPRs that have previously been identified and functionally characterized confirming that the receptor isolated in this study is an ortholog of other insect ACP receptors17,18,22,25. Predicted ACPRs from other mosquito species including, Anopheles darlingi, Culex pipiens, and the Asian tiger mosquito Aedes albopictus also cluster closely to the A. gambiae and A. aegypti ACP receptors.
Figure 3.
Phylogenetic relationship of adipokinetic hormone receptors (AKHR,  ), corazonin receptors (CRZR,
), corazonin receptors (CRZR,  ) and adipokinetic hormone/corazonin-related peptide receptors (ACPR,
) and adipokinetic hormone/corazonin-related peptide receptors (ACPR,  ) from insects. Tree was constructed using the neighbour-joining analysis (with 1000 bootstrap replicates). Branch lengths indicate the number of amino acid substitutions per site and numbers adjacent to nodes denotes the percentage support for the clustering of the related sequences in that particular clade. Receptor protein sequences are labelled by species name and identified with their GenBank accession number. The AedaeACPR-I receptor cloned in this study is denoted in red text. The human gonadotropin releasing hormone receptor isoform 1 (GenBank: NP_000397) was included in the analysis and designated as the outgroup.
) from insects. Tree was constructed using the neighbour-joining analysis (with 1000 bootstrap replicates). Branch lengths indicate the number of amino acid substitutions per site and numbers adjacent to nodes denotes the percentage support for the clustering of the related sequences in that particular clade. Receptor protein sequences are labelled by species name and identified with their GenBank accession number. The AedaeACPR-I receptor cloned in this study is denoted in red text. The human gonadotropin releasing hormone receptor isoform 1 (GenBank: NP_000397) was included in the analysis and designated as the outgroup.
A heterologous receptor functional assay involving CHO-K1 cells was used to validate the cloned receptor as a bona fide ACP receptor. Indeed, the AedaeACPR-I was dose-dependently activated by AedaeACP (EC50 = 1.025 × 10−8 M) (Fig. 4a), confirming the proposed identity of the receptor based on phylogenetic analysis. Kinetic analysis of receptor activation demonstrated maximal luminescence response was evident over the first five seconds following application of the ACP peptide, indicative of an immediate and transient elevation of intracellular calcium levels elicited through activation of the ACP receptor (Fig. 4b). Our results also confirm the specificity of the ACP receptor for the ACP peptide alone (see Table 2), since no detectable luminescence indicative of receptor activation was observed in response to the closely related peptides, AedaeAKH and AedaeCRZ, or other tested peptides, specifically AedaeCAPA-1 and pyrokinin-1 (AedaePK1), which share no structural similarity to AedaeACP. Our findings in this study are consistent with past reports, since similar binding specificity of ACP receptors has been observed previously in T. castaneum, A. gambiae, and R. prolixus with EC50 values reported in the low nanomolar range17,25. Additionally, previous research in the aforementioned insects have also observed that the AKH receptors are not activated by ACP or corazonin peptides, and similarly, the corazonin receptors are not activated by ACP or AKH peptides17,25,43,44. Thus, consistent with these previous observations, we determined that although these neuropeptide systems are structurally and evolutionarily related, they are indeed independent of one another and do not exhibit any cross talk in A. aegpyti. Notably, however, previous studies in B. mori have revealed that high concentrations of Bommo-ACP (previously referred to as AKH3) resulted in the activation of Bommo-AKHR whereas sensitivity to its natural AKH ligand was approximately 100-fold higher23. Similarly, high doses of the AKH peptides in B. mori, Bommo-AKH1 and Bommo-AKH2, were also found to activate putative B. mori ACPRs (A28 and A29), albeit at significantly higher concentrations than ACP22.
Figure 4.
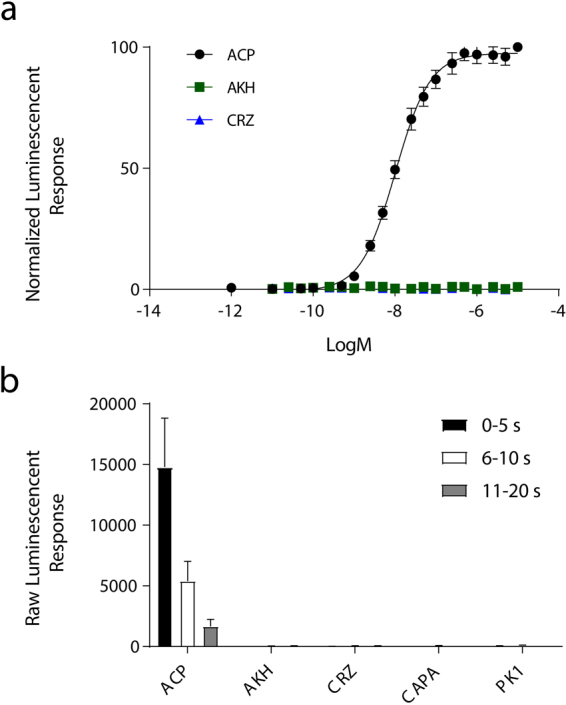
Functional heterologous receptor assay of CHO-K1 aequorin cells transiently expressing the AedaeACPR-I. (a) Dose dependent effect on the bioluminescence response (mean 0–15 s) after the addition of between 10−12–10−5 M doses of A. aegypti ACP, AKH and CRZ peptides. Luminescence is normalized to the BSA control and plotted relative to the maximal response (10−5 M). The EC50 for AedaeACP is 1.025 × 10−8 M. No receptor activation was detected when challenged with AedaeAKH and AedaeCRZ or AedaeCAPA1 and AedaePK1 (not shown). (b) Kinetics of the bioluminescence response measured between 0–5 s, 6–10 s, and 11–20 s time intervals, following the addition of 1 × 10−6 M of the above peptides normalized to vehicle control (BSA media). Luminescence is normalized to the BSA control and plotted relative to the maximal response (10−5 M). Data represent the mean ± standard error (n = 4).
Table 2.
Structure of peptides tested in the heterologous receptor functional assay and summary of activity in eliciting a luminescent response.
| Peptide Name | Peptide Sequence | EC50 (nM)a |
|---|---|---|
| AedaeACP | pQVTFSRDWNAa | 10.25 |
| AedaeAKH | pQLTFTPSWa | NAb |
| AedaeCRZ | pQTFQYSRGWNTNa | NAb |
| AedaeCAPA1 | GPTVGLFAFPRVa | NAb |
| AedaeCAPA-PK1 | AGNSGANSGMWFGPRLa | NAb |
aEC50 values are the averages of multiple independent biological replicates involving CHOK1-aeq cells transiently expressing AedaeACPR-I.
bNo activity (NA) detected when tested with peptide titres up to a maximum of 10 µM.
In comparison to the A. aegypti genome, a number of single nucleotide polymorphisms (SNP) were observed across the entire cDNA sequence. Of those occurring within the open reading frame, only one SNP (nucleotide 1924, found within the seventh exon, which corresponds to the C-terminus of the receptor) results in a different amino acid at residue Ile472, compared to the Asn472 predicted by the A. aegypti genome. Modification of the isoleucine residue (Ile472) obtained in our cDNA to the genome consistent asparagine residue (Asn472) resulted in no change to receptor activation by its endogenous ACP ligand, as determined by equal luminescent response by both the cloned AedaeACPR-I and the mutated AedaeACPR-I-N472I (Fig. S2). No luminescence signals were obtained in response to any of the tested peptides in untransfected cells, or cells transfected with empty pcDNA 3.1+ vector (data not shown).
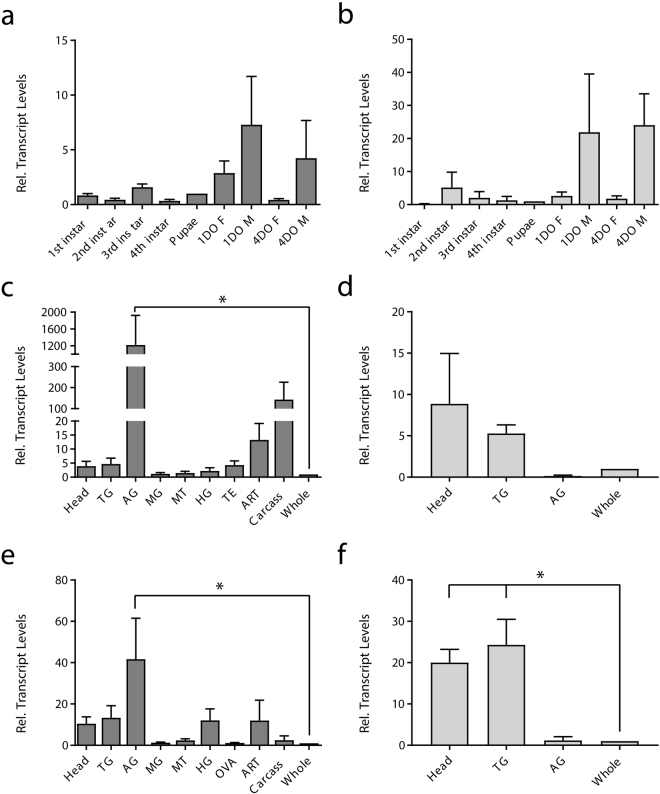
Next, utilizing RT-qPCR, we investigated the molecular expression of the ACP signaling system during development and in individual tissues of adult A. aegypti. Although we identified three transcript variants, only the ACPR-I transcript yields a complete receptor protein which we functionally deorphanized, and so expression profiles were determined only for this transcript variant (see methods). Developmental expression profiling revealed enrichment of both ACPR-I (Fig. 5a) and ACP (Fig. 5b) transcripts following the transition from pupal to adult stages. In particular, one-day and four-day old male A. aegypti had the highest levels of ACP and ACPR-I transcript abundance. Similar findings for the ACP receptor were observed in R. prolixus17,25; however, in contrast, ACPR transcript levels in T. castaenum were highest in late embryonic and early larval stages and decreased thereafter as the beetle progressed in development17,25. In A. aegypti, the observed enrichment of the ACP and ACPR-I transcripts could be indicative of a post-eclosion function for the ACP system.
Figure 5.
Transcript expression pattern of ACPR-I and ACP during post-embryonic development and in specific tissues of four-day old adult A. aegypti. Temporal expression of ACPR-I (a) and ACP (b) transcript is analyzed across developmental stages of the mosquito with expression shown relative to transcript levels in pupa. Spatial expression is analyzed in various tissues from four-day old adult males, ACPR-I (c) and ACP (d), and females, ACPR-I (e) and ACP (f) with transcript abundance shown relative to levels in whole body adult mosquitoes. Abbreviations: thoracic ganglia (TG), abdominal ganglia (AG), midgut (MG), Malpighian tubules (MT), hindgut (HG), ovaries (OVA), testes (TE), accessory reproductive tissue (ART). Data represent mean ± standard error, *denotes significance (p < 0.05) as determined by a one-way ANOVA, Dunnett’s multiple comparisons test.
Spatial transcript expression profiles of A. aegypti ACPR aimed to reveal potential functional roles for ACP and its receptor by determining prospective target tissues. The ACP receptor (ACPR-I) was found to be significantly enriched in the abdominal ganglia of both male (Fig. 5c) and female (Fig. 5e) A. aegypti when compared relative to expression in the whole adult (males, p = 0.0025 and for females, p = 0.0016). ACPR-I transcript was also observed in the carcass, accessory reproductive tissue, testes, head and thoracic ganglia of adult male mosquitoes, although not significantly enriched as was found in the abdominal ganglia (Fig. 5c). Similarly, ACPR-I transcript was also found in the head, thoracic ganglia, hindgut, and accessory reproductive tissues of adult female A. aegypti (Fig. 5e). Enrichment of the ACP receptor transcript in the nervous system is not surprising, since expression of the transcripts encoding the ACP receptor and peptide have been described in the nervous system of other insects. Specifically, in fifth instar and adult R. prolixus, ACPR transcript was found to be enriched in the central nervous system and the corpus cardiacum /corpora allata (CC/CA) complex25. ACPR expression in T. castaneum was revealed to be greatest in the brain in comparison to the torso (i.e. body minus the head)17. AedaeACP transcript was detected in the central nervous system, where it was enriched in the brain and thoracic ganglia of male (Fig. 5d) and significantly enriched in the head (p = 0.0127) and thoracic ganglia (p = 0.004) of female mosquitoes (Fig. 5f). Consistent with our quantified ACP tissue-specific expression profile, ACP transcripts in A. aegypti and A. gambiae were detected solely in head and thorax body segments of adult mosquitoes20,21.
Expression of A. aegypti ACPR-I within the central nervous system suggests that ACP may be functioning as a neuromodulator and/or a neurotransmitter. This possibility is supported by the extensive varicose immunoreactive staining of ACP in the central nervous system of T. castaneum first instar larvae, where a neurosecretory role was suggested17. Specifically, immunoreactivity (IR) was observed in three to four neurons in the brain, the central brain neuropil with projections from the brain descending into the suboesophageal ganglion (SOG), thoracic ganglia, and abdominal ganglia, with no projections observed exiting the nervous system17. Immunocytochemistry using an antiserum against D. melanogaster AKH, which recognizes both AKH and ACP in A. gambiae and A. aegypti20,21, revealed immunoreactivity throughout the mosquito nervous system. In both A. aegypti and A. gambiae, AKH-like immunoreactivity was observed in two pairs of lateral neurosecretory cells in the brain, but was explained by the authors of this study to represent ACP-producing neurons, since AKH synthesis and storage is restricted to the corpus cardiacum20,21. Within the fused thoracic ganglia of adult A. aegypti and A. gambiae, AKH-like immunoreactivity was observed within at least one to a few cells within the ventral region of the pro- and mesothoracic ganglia20,21. Thoracic extracts were negative for ACP-like activity in A. gambiae20,21, however, expression of the AKH transcript in adult male A. aegypti is absent in the head and thorax region20,21; thus, it is unclear if the cells detected previously within the thoracic ganglia are ACP- or AKH-producing neurons in mosquitoes although our data indicate that the A. aegypti ACP transcript is prominently expressed in both the brain and thoracic regions of the nervous system. In R. prolixus, ACP-like immunoreactivity is observed in two bilaterally paired cell bodies at the anterior portion of the protocerebrum near the optic lobes, and one bilateral pair of cell bodies medially positioned in the protocerebrum10. Considering the extensive immunohistochemical distribution of ACP throughout the nervous system of insects16,19,20, the prominent expression of ACP transcript in the brain and thoracic ganglia along with the significant enrichment of ACPR-I within the abdominal ganglia of adult A. aegypti strongly supports that ACP may be functioning centrally within the nervous system. In L. migratoria ACP was identified in the storage lobe of the CC, in contrast to the glandular lobe where AKH is found, suggesting synthesis of this neuropeptide within neurosecretory cells of the brain18. Furthermore, it was previously suggested that given ACPR was found in the CC/CA complex in R. prolixus, ACP may be involved in the regulation of other hormones in a manner similar to its distant vertebrate homolog, GnRH25.
We also determined A. aegypti ACPR-I expression is not restricted to nervous tissue since transcript expression was detected in other tissues/organs including the female hindgut (Fig. 5e) and male carcass (Fig. 5c). ACPR expression in the hindgut, the primary site of reabsorption of ions and metabolites45, was unexpected since neither AKH nor CRZ have been found to regulate hydromineral balance. Thus, this possible function for ACP on the hindgut will require further investigation. Detection of the ACPR transcript in the carcass, which includes the body wall musculature and fat body, suggests that ACP and AKH may share a lipid mobilizing function. However, this possibility is unlikely since ACP was shown to have no influence on lipid or carbohydrate metabolism in female A. gambiae nor did it influence energy stores in male insects of L. migratoria or P. americana46. Interestingly, contrary to our predictions, both spatial and temporal expression profiles reveal greater expression of ACP and ACPR in adult males compared to females, which is consistent with an earlier microarray analysis in A. gambiae that determined higher ACP expression in adult males, compared to adult female and last instar larvae47. There is no clear explanation for such a sex-specific difference in ACP and ACPR transcript expression, however similar to our findings, male D. melanogaster express greater levels of the AKH receptor than their female counterparts8. ACPR-I transcript expression was also observed in the accessory reproductive tissues of both male and female A. aegypti. Expression of ACPR in reproductive tissue has also been observed in R. prolixus25. Perhaps, in addition to structural homology between ACPR and GnRHR, there is a yet undiscovered functional conservation between these two signaling systems. Furthermore, in Gryllus bimaculatus, pharmacologically elevating AKH titre through injections resulted in a significantly lower egg production48. In Caenorhabditis elegans, knockdown of the AKH-GnRH peptide and GnRH receptor resulted in reduced progeny in early worms49. Also, in Glossina morsitans, knockdown of the AKHR transcript resulted in higher levels of whole body lipids and, in pregnant flies, the inability to utilize lipid reserves resulted in delayed larval development and thus reproductive disruption50. Whether these effects on reproduction in these organisms are a direct result of signalling involving the AKH or GnRH receptor or AKH peptide remains unclear. Additionally, analysis of seminal fluid protein content of A. albopictus revealed the AKH peptide as one of the proteins transferred from males to female mosquitoes during mating51. Recently, CRZR transcript expression in R. prolixus was also observed in male and female reproductive tissues, which suggests some potentially overlapping reproductive target tissues in insects44.
Currently, no definitive function for ACP has been determined and functional studies in other insects have revealed that ACP does not influence energy mobilization and so does not duplicate the actions of AKH10,46. Additionally, ACP failed to increase heart-beat frequency, suggesting that the physiological actions of ACP does not mirror the most established function of CRZ10. Further studies are necessary to unravel the function of the ACP system, which could include methods aimed at knockdown of the ACP peptide or receptor.
Electronic supplementary material
Acknowledgements
Research conducted in this study was supported through new-investigator institutional start-up funds, an NSERC Discovery Grant and Petro Canada Young Innovator Award to J.P.P.
Author Contributions
Conceived and designed the experiments: A.W. and J.P.P. Performed the experiments and analyzed data: A.W. and J.P.P. Wrote the paper: A.W. and J.P.P.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20517-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klavdieva MM. The history of neuropeptides I. Front Endocrinol. 1995;16:293–321. doi: 10.1006/frne.1995.1011. [DOI] [PubMed] [Google Scholar]
- 2.Nässel DR. Neuropeptides in the insect brain: a review. Cell Tissue Res. 1993;273:1–29. doi: 10.1007/BF00304608. [DOI] [PubMed] [Google Scholar]
- 3.Diederen JHB, Oudejans RCHM, Harthoorn LF, Van Der Horst DJ. Cell biology of the adipokinetic hormone-producing neurosecretory cells in the locust corpus cardiacum. Microsc Res Tech. 2002;56:227–236. doi: 10.1002/jemt.10026. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz MW, Gäde G. Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr Comp Biol. 2009;49:380–392. doi: 10.1093/icb/icp019. [DOI] [PubMed] [Google Scholar]
- 5.Gäde G, Marco HG. Structure, function and mode of action of select arthropod neuropeptides. Stud Nat Prod Chem. 2006;33:69–139. doi: 10.1016/S1572-5995(06)80026-8. [DOI] [Google Scholar]
- 6.Staubli F, et al. Molecular identification of the insect adipokinetic hormone receptors. Proc Natl Acad Sci USA. 2002;99:3446–3451. doi: 10.1073/pnas.052556499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park Y, Kim Y-JJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA. 2002;99:11423–11428. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauser F, Søndergaard L, Grimmelikhuijzen CJ. Molecular cloning, genomic organization and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to gonadotropin-releasing hormone receptors for vertebrates. Biochem Biophys Res Commun. 1998;249:822–828. doi: 10.1006/bbrc.1998.9230. [DOI] [PubMed] [Google Scholar]
- 9.Veenstra JA. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett. 1989;250:231–234. doi: 10.1016/0014-5793(89)80727-6. [DOI] [PubMed] [Google Scholar]
- 10.Patel H, Orchard I, Veenstra JA, Lange AB. The distribution and physiological effects of three evolutionarily and sequence-related neuropeptides in Rhodnius prolixus: Adipokinetic hormone, corazonin and adipokinetic hormone/corazonin-related peptide. Gen Comp Endocrinol. 2014;203:307–314. doi: 10.1016/j.ygcen.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Hillyer JF, Estévez-Lao TY, Funkhouser LJ, Aluoch VA. Anopheles gambiae corazonin: Gene structure, expression and effect on mosquito heart physiology. Insect Mol Biol. 2012;21:343–355. doi: 10.1111/j.1365-2583.2012.01140.x. [DOI] [PubMed] [Google Scholar]
- 12.Predel R, Neupert S, Russell WK, Scheibner O, Nachman RJ. Corazonin in insects. Peptides. 2007;28:3–10. doi: 10.1016/j.peptides.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Tawfik aI, et al. Identification of the gregarization-associated dark-pigmentotropin in locusts through an albino mutant. Proc Natl Acad Sci USA. 1999;96:7083–7087. doi: 10.1073/pnas.96.12.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y-J, et al. Corazonin receptor signaling in ecdysis initiation. Proc Natl Acad Sci USA. 2004;101:6704–6709. doi: 10.1073/pnas.0305291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Hua YJ, Roller L, Tanaka S. Corazonin reduces the spinning rate in the silkworm, Bombyx mori. J Insect Physiol. 2002;48:707–714. doi: 10.1016/S0022-1910(02)00094-X. [DOI] [PubMed] [Google Scholar]
- 16.Gospocic J, et al. The neuropeptide corazonin controls social behavior and caste identity in ants. Cell. 2017;170:748–759.e12. doi: 10.1016/j.cell.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen KK, et al. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J Biol Chem. 2010;285:10736–10747. doi: 10.1074/jbc.M109.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegert KJ. Locust corpora cardiaca contain an inactive adipokinetic hormone. FEBS Lett. 1999;447:237–240. doi: 10.1016/S0014-5793(99)00299-9. [DOI] [PubMed] [Google Scholar]
- 19.Belmont M, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJP. Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae. Biochem Biophys Res Commun. 2006;344:160–165. doi: 10.1016/j.bbrc.2006.03.117. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann C, Merzendorfer H, Gäde G. The adipokinetic hormone system in Culicinae (Diptera: Culicidae): Molecular identification and characterization of two adipokinetic hormone (AKH) precursors from Aedes aegypti and Culex pipiens and two putative AKH receptor variants from A. aegypti. Insect Biochem Mol Biol. 2009;39:770–781. doi: 10.1016/j.ibmb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann C, Brown MR. Adipokinetic hormones in the African malaria mosquito, Anopheles gambiae: Identification and expression of genes for two peptides and a putative receptor. Insect Biochem Mol Biol. 2006;36:466–481. doi: 10.1016/j.ibmb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, et al. Identification and functional characterization of two orphan G-protein-coupled receptors for adipokinetic hormones from silkworm Bombyx mori. J Biol Chem. 2011;286:42390–42402. doi: 10.1074/jbc.M111.275602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu C, et al. Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori. FEBS Lett. 2009;583:1463–1468. doi: 10.1016/j.febslet.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, B. et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res 113–122 (2008). [DOI] [PMC free article] [PubMed]
- 25.Zandawala M, Haddad AS, Hamoudi Z, Orchard I. Identification and characterization of the adipokinetic hormone/corazonin-related peptide signaling system in Rhodnius prolixus. FEBS J. 2015;282:3603–3617. doi: 10.1111/febs.13366. [DOI] [PubMed] [Google Scholar]
- 26.Barón OL, Ursic-Bedoya RJ, Lowenberger C, Ocampo CB. Differential gene expression from midguts of refractory and susceptible lines of the mosquito, Aedes aegypti, infected with Dengue-2 virus. J Insect Sci. 2010;10:41. doi: 10.1673/031.010.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocco DA, Kim DH, Paluzzi JV. Immunohistochemical mapping and transcript expression of the GPA2/GPB5 receptor in tissues of the adult mosquito, Aedes aegypti. Cell Tissue Res. 2017;369:313–330. doi: 10.1007/s00441-017-2610-3. [DOI] [PubMed] [Google Scholar]
- 28.Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in Genie. J Comput Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 29.Dobson L, et al. CCTOP: a Consensus Constrained TOPology prediction web server. Nucleic Acids Res. 2015;43:W408–W412. doi: 10.1093/nar/gkv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 32.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Bioinformatics. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 33.Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984;308:241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- 34.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 35.Paluzzi J-PV, et al. Investigation of the potential involvement of eicosanoid metabolites in anti-diuretic hormone signaling in Rhodnius prolixus. Peptides. 2012;34:127–134. doi: 10.1016/j.peptides.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Iredale PA, Hill SJ. Increases in intracellular calcium via activation of an endogenous P2-purinoceptor in cultured CHO-K1 cells. Br J Pharmacol. 1993;110:1305–1310. doi: 10.1111/j.1476-5381.1993.tb13960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michel AD, Chessell IP, Hibell AD, Simon J, Humphrey PPA. Identification and characterization of an endogenous P2X7 (P2Z) receptor in CHO-K1 cells. Br J Pharmacol. 1998;125:1194–201. doi: 10.1038/sj.bjp.0702205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paluzzi J-P, Vanderveken M, O’Donnell MJ. The heterodimeric glycoprotein hormone, GPA2/GPB5, regulates ion transport across the hindgut of the adult mosquito, Aedes aegypti. PLoS One. 2014;9:1–14. doi: 10.1371/journal.pone.0086386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2014;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirzadegan T, Benkö G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42:2759–67. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Audet M, Bouvier M. Restructuring G-protein- coupled receptor activation. Cell. 2012;151:14–23. doi: 10.1016/j.cell.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–67. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zandawala M, Hamoudi Z, Lange AB, Orchard I. Adipokinetic hormone signalling system in the Chagas disease vector, Rhodnius prolixus. Insect Mol Biol. 2015;24:264–276. doi: 10.1111/imb.12157. [DOI] [PubMed] [Google Scholar]
- 44.Hamoudi Z, Lange AB, Orchard I. Identification and characterization of the corazonin receptor and possible physiological roles of the corazonin-signaling pathway in Rhodnius prolixus. Front Neurosci. 2016;10:1–12. doi: 10.3389/fnins.2016.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coast GM, Orchard I, Phillips JE, Schooley DA. Insect diuretic and antidiuretic hormones. Adv Insect Physiol. 2002;29:279–409. doi: 10.1016/S0065-2806(02)29004-9. [DOI] [Google Scholar]
- 46.Kaufmann C, Brown MR. Regulation of carbohydrate metabolism and flight performance by a hypertrehalosaemic hormone in the mosquito Anopheles gambiae. J Insect Physiol. 2008;54:367–377. doi: 10.1016/j.jinsphys.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marinotti O, Nguyen QK, Calvo E, James AA, Ribeiro JMC. Microarray analysis of genes showing variable expression following a blood meal in Anopheles gambiae. Insect Mol Biol. 2005;14:365–373. doi: 10.1111/j.1365-2583.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 48.Lorenz MW. Adipokinetic hormone inhibits the formation of energy stores and egg production in the cricket Gryllus bimaculatus. Comp Biochem and Physiol - B Bioch and Mol Biol. 2003;136:197–206. doi: 10.1016/S1096-4959(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 49.Lindemans M, et al. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106:1642–7. doi: 10.1073/pnas.0809881106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attardo GM, et al. Analysis of lipolysis underlying lactation in the tsetse fly. Glossina morsitans. Insect Biochem Mol Biol. 2012;42:360–370. doi: 10.1016/j.ibmb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boes KE, et al. Identification and characterization of seminal fluid proteins in the Asian tiger mosquito, Aedes albopictus. PLoS Negl Trop Dis. 2014;8:e2946. doi: 10.1371/journal.pntd.0002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.