Figure 7.
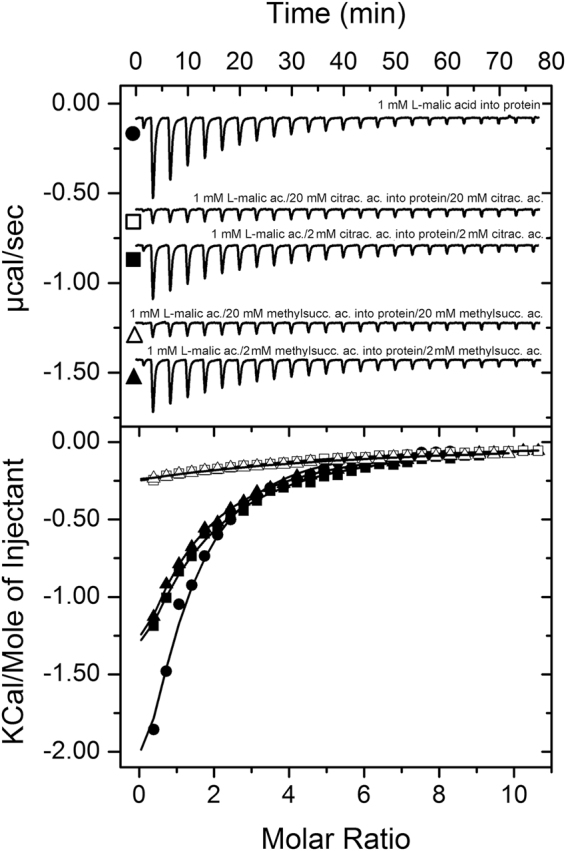
Attractants and antagonists compete for binding at PA2652-LBD in vitro. Isothermal titration calorimetry analysis of the binding of L-malic acid to PA2652-LBD in the absence and presence of 2 or 20 mM of the antagonists, citraconic and D,L-methylsuccinic acids. Upper panel: Titration raw data for the injection of 9.6 μl aliquots of 1 mM of L-malic acid into 20 μM of protein in the absence and presence of antagonists (present both in the injector syringe and sample cell). Lower panel: Integrated, dilution heat corrected and concentration normalized peak areas fitted with the “One binding site” model of ORIGIN. The apparent binding constants are listed in Supplementary Table S2.
