ABSTRACT
Microbial partners play important roles in the biology and ecology of animals. In insects, maternally transmitted symbionts are especially common and can have host effects ranging from reproductive manipulation to nutrient provisioning and defense against natural enemies. In this study, we report a genus-wide association of Myrmica ants with the inherited bacterial symbiont Spiroplasma. We screen Myrmica ants collected from the wild, including the invasive European fire ant, Myrmica rubra, and find an extraordinarily high prevalence of this symbiont—8 of 9 species, 42 of 43 colonies, and 250 of 276 individual workers harbored Spiroplasma—only one host species was uninfected. In our screens, each host species carried a distinct Spiroplasma strain, and none were infected with more than one strain. All symbionts belong to the citri clade, allied most closely with pathogenic strains of Spiroplasma infecting corn crops and honeybees, and there is strong evidence of host-symbiont persistence across evolutionary time scales. Genome sequencing of two Spiroplasma symbionts revealed candidate genes that may play a part in the symbiosis, a nutrient transporter absent from other Spiroplasma strains, and a ribosome-inactivating protein previously implicated in parasite defense. These results together suggest long-term, likely mutualistic, relationships atypical of Spiroplasma-insect associations with potential significance for broad ecological interactions with Myrmica.
IMPORTANCE Animal-associated microbial symbionts can dramatically affect the biology of their hosts. The identification and characterization of these intimate partnerships remain an essential component of describing and predicting species interactions, especially for invasive host species. Ants perform crucial ecological functions as ecosystem engineers, scavengers, and predators, and ants in the genus Myrmica can be aggressive resource competitors and reach high densities in their native and invaded habitats. In this study, a novel symbiosis is identified between Myrmica ants and the facultative bacterial symbiont Spiroplasma. Broad host distribution, high frequencies of infection, and host-symbiont codivergence over evolutionary time scales, an uncommon feature of Spiroplasma associations, suggest an important likely mutualistic interaction. Genome sequencing identified highly divergent gene candidates that may contribute to Spiroplasma's role as a possible defensive or nutritional partner in Myrmica.
KEYWORDS: defensive symbiosis, facultative symbiont, mutualism, ribosome-inactivating proteins, symbiosis
INTRODUCTION
It is now well established that most insects harbor maternally inherited bacterial endosymbionts that play critical roles in the ecology and evolution of their hosts (1). Insect lineages that feed exclusively on nutrient-poor diets, such as plant sap or animal blood, typically host obligate nutritional endosymbionts that provide essential vitamins and amino acids. These obligate endosymbionts are often housed in specialized symbiont organs and show patterns of strict and ancient codiversification with their hosts.
More common still are facultative inherited symbionts, of which the best known is Wolbachia (2). While these symbionts are transmitted almost exclusively through females over ecological time scales they very rarely cospeciate with their hosts and instead repeatedly colonize new host lineages over evolutionary time scales via horizontal transmission. Although not essential for host survival or reproduction, many facultative inherited symbionts increase host fitness under certain conditions, for example, by protecting their hosts against natural enemies or environmental stresses (3–8). Others manipulate their host's reproduction to increase the frequency of symbiont-infected females (9–13). Five bacterial lineages are particularly widespread as facultative symbionts of insects. In addition to Wolbachia, these are Arsenophonus, Cardinium, Rickettsia, and Spiroplasma (14, 15). Initial surveys have found that these bacteria infect ∼5 to 30% of insect species—this represents millions of infected species. However, most insect lineages have been poorly sampled, and even when infections have been reported, it is often not understood how these facultative symbionts affect host fitness or persist in host populations.
Spiroplasma is an incredibly diverse genus of bacteria that infect arthropods, with a wide range of fitness effects and transmission strategies (16). Many Spiroplasma strains are pathogenic, including pathogens of bees, crayfish, and plants (17–20). Even more prevalent are horizontally transmitted gut commensals that have been isolated from a wide range of insects, including beetles and flies (21, 22). Finally, maternal transmission has evolved independently in a number of Spiroplasma lineages. Vertically transmitted Spiroplasma can be found both inside and outside cells, often at high densities in insect hemolymph, as well as in ovarian tissues (23). While the effects of most vertically transmitted Spiroplasma strains are not known, a number of strains manipulate their host's reproduction by killing male embryos; male-killing Spiroplasma strains have been documented in butterflies, planthoppers, beetles, flies, and lacewings (10, 11, 24–26). Some Spiroplasma strains protect their hosts against natural enemies, with strains that infect aphids providing protection against pathogenic fungi (27) and strains that infect Drosophila flies protecting against parasitic wasps and nematodes (4, 8). Recent studies have implicated a diverse arsenal of toxins called ribosome-inactivating proteins (RIPs) in Drosophila defense (28, 29). The identification of a Spiroplasma-encoded RIP transcript in the publically available transcriptome of the European invasive fire ant Myrmica rubra motivated a closer examination of the relationship between Spiroplasma and this ant genus in the present study.
Although Spiroplasma infects a wide range of arthropods, few studies have examined a specific group of hosts in detail. The best-studied inherited Spiroplasma strains are those that infect Drosophila. At least 18 species have been found to harbor Spiroplasma (8, 30, 31), with infection frequencies ranging from less than 5% to greater than 85% (32, 33). Drosophila flies have been independently colonized by five different lineages of inherited Spiroplasma from the citri, poulsonii, ixodetis, and tenebrosa clades (30). In this study, ant species in the genus Myrmica were surveyed for Spiroplasma. This genus also appears to be a hot spot for Spiroplasma infection, with all species except one infected at high frequencies. However, unlike those of Drosophila, Spiroplasma strains infecting Myrmica are all members of the citri clade, and there is a strong phylogenetic signal suggesting persistent host-symbiont associations across evolutionary time scales.
RESULTS
Spiroplasma symbionts are widespread in Myrmica.
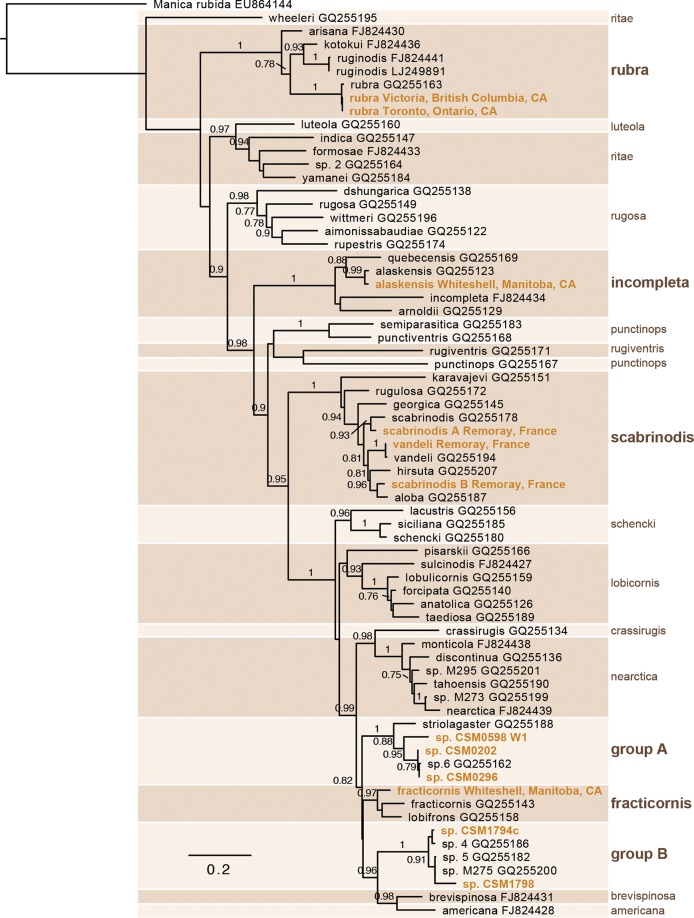
Nine species of Myrmica, broadly distributed across the genus (Fig. 1), were screened for Spiroplasma by PCR amplification of the ftsZ gene; all but one were positive (Table 1). Spiroplasma genes were also detected in all three publicly available Myrmica transcriptomes (M. rubra, M. ruginodis, and M. sulcinodis [NCBI BioProject number PRJDB4088]). Among the screened species, the prevalence of infection was high, with 250 of 276 individuals and 42 of 43 colonies testing positive. COI failed to amplify or yield quality sequences from nine of the 284 DNA extractions, and these samples were excluded from prevalence calculations. In one case, ftsZ amplified from a Myrmica sp. sample in which COI failed to amplify, and this sample was conservatively excluded from subsequent analysis. For the two best-sampled species, M. rubra and M. scabrinodis (two mtDNA haplotypes), the infection frequencies were 86% and 96%, respectively, and all colonies were infected. Infection frequencies were similarly high in juvenile stages, with 9 of 10 larvae and 8 of 9 pupae from one M. scabrinodis colony testing positive. Many of the species in our data set are represented by a single colony or individual, yet in most cases, Spiroplasma was consistently detected despite limited sampling; however, a high prevalence for these species should not be assumed until it can be demonstrated through a similarly thorough sampling effort.
FIG 1.
Diverse Myrmica ants harbor Spiroplasma. Shown is a maximum-likelihood phylogram of mitochondrially encoded cytochrome oxidase subunit I (COI) nucleotide sequences of ants in the genus Myrmica. Gold type indicates taxa screened for Spiroplasma. Species groups are designated by alternately shaded boxes and labeled at the right side of the phylogram. Bold type marks a species group containing one or more screened taxa. Branches are labeled with SH-like approximate likelihood ratio test scores greater than 0.75.
TABLE 1.
Myrmica species or species groups screened for Spiroplasma
| Myrmica species or group | No. of individuals/no. of colonies: |
Frequency (%) | |
|---|---|---|---|
| Screened | Positive | ||
| M. alaskensis | 16/1 | 16/1 | 100.0 |
| M. fracticornis | 8/1 | 0/0 | 0.0 |
| M. rubra | 68/12 | 58/12 | 85.2 |
| M. scabrinodis mtDNA type A | 37/6 | 34/6 | 91.9 |
| M. scabrinodis mtDNA type B | 126/16 | 123/16 | 97.6 |
| sp. 1 (group A, CSM0598 W1) | 1/1 | 1/1 | 100.0 |
| sp. 2 (group A, CSM0202 and CSM0296) | 4/2 | 4/2 | 100.0 |
| sp. 3 (group B, CSM1794c) | 1/1 | 1/1 | 100.0 |
| sp. 4 (group B, CSM1798) | 2/1 | 1/1 | 50.0 |
| M. vandeli | 13/2 | 12/2 | 92.3 |
To determine where Spiroplasma infection is localized, DNA extractions from the heads, thoraces, gasters, and legs of adult Myrmica vandeli and M. scabrinodis were screened; all tissue types were positive, indicating Spiroplasma is present in the hemolymph and is not restricted to the gut.
Spiroplasma-host specificity and evolutionary relationships.
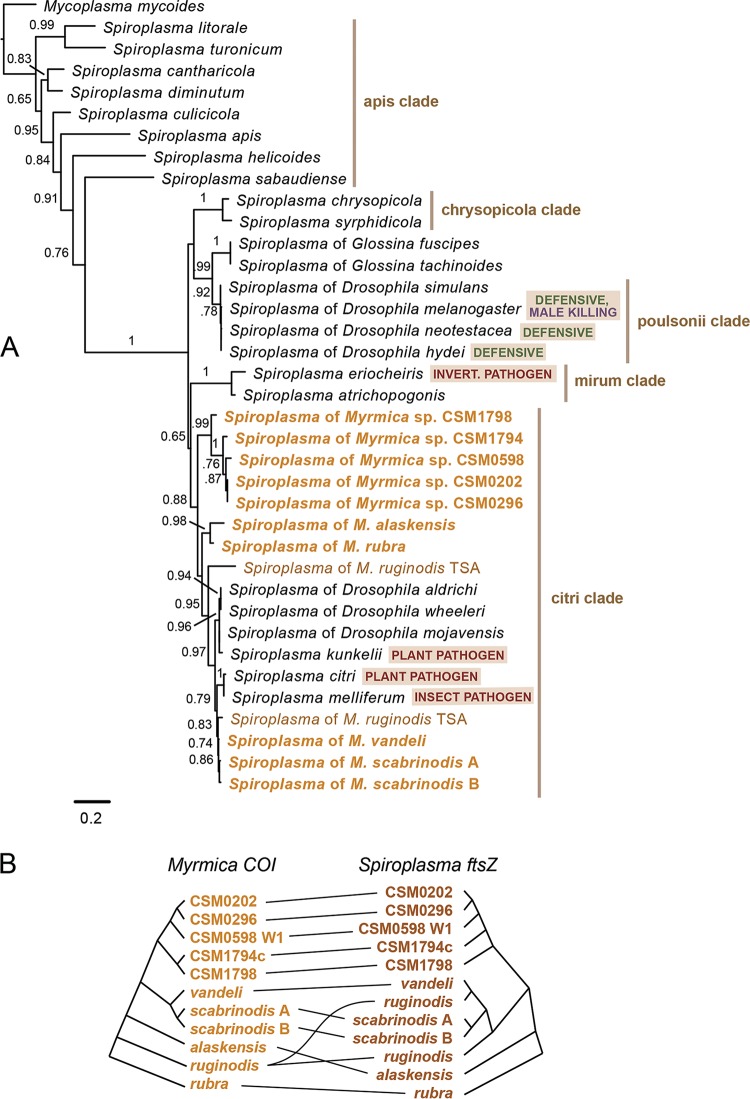
A phylogenetic analysis of symbiont ftsZ sequences places all of the Myrmica Spiroplasma strains in the citri clade (Fig. 2A), although they are not monophyletic. No Spiroplasma strain was shared between species. Two distinct ftsZ sequences were recovered from the published transcriptome of M. ruginodis, suggesting a coinfection; however, this was not examined in greater detail, as M. ruginodis samples were not screened. Myrmica Spiroplasma strains form three clades that appear to correspond with host species groups: one with members of the scabrinodis group, one with members of the fracticornis group and allies, and a third with M. rubra and M. alaskensis. Unlike the Spiroplasma strains of Myrmica, those of other ants are more broadly distributed throughout the genus Spiroplasma (see Fig. S1 in the supplemental material). ParaFit was used to perform a global test of host-symbiont codivergence among all 10 distinct host lineages that were screened plus M. ruginodis. The null hypothesis of an independent host and symbiont evolution was not rejected at a significance threshold of 0.05 (P = 0.07) (Fig. 2B). The exclusion of M. ruginodis and its dual Spiroplasma strains resulted in a rejection of the null hypothesis, though at a marginally significant P value of 0.02.
FIG 2.
Spiroplasma strains in Myrmica belong to the citri clade. (A) Maximum-likelihood phylogram of cell division protein ftsZ nucleotide sequences from Spiroplasma. Gold type indicates taxa amplified from Myrmica ants in this study. Branches are labeled with SH-like approximate likelihood ratio test scores of 0.65 or higher. (B) Tanglegram depicting the pattern of cophylogeny between host and symbiont gene trees. Horizontal black lines between trees connect host taxa to symbiont taxa. Codivergence is not statistically significant as tested by ParaFit (P = 0.07).
The most thorough sampling was from Lac de Remoray, France, where 22, 2, and 3 colonies of M. scabrinodis, M. vandeli, and Formica picea were collected within meters of each other. Myrmica scabrinodis contained two distinct mitochondrial haplotypes (96.2% similar at COI), and each of these haplotypes harbored its own Spiroplasma ftsZ haplotype (99.2% similar at ftsZ). Sixteen colonies had one mitochondrial haplotype, six had the other, and no colony had both. Sanger sequencing of a fragment of the long-wavelength rhodopsin gene, as well as our Illumina genomic DNA sequencing, confirmed that these two mitochondrial haplotypes are one species (i.e., there were no differences in nuclear genes). The two M. vandeli colonies harbored a distinct Spiroplasma strain that was 99.0 to 99.2% similar at ftsZ to the Spiroplasma in M. scabrinodis. Spiroplasma was absent from the three Formica picea colonies (n = 26 individuals), further highlighting the absence of lateral transfer of Spiroplasma symbionts among microsympatric hosts.
Lastly, two of the M. scabrinodis colonies were initially keyed as M. martini, a species that was only recently described on the basis of complex morphometrics (34), with no clear morphological features distinguishing it from M. scabrinodis (and with the authors' discriminant function misclassifying 10% of individuals). Molecular data from both colonies—mitochondrial and nuclear loci, as well as the symbiont locus—are identical to those from the other 14 M. scabrinodis haplotype B colonies in our study, suggesting M. martini is not a valid species.
Genome content of Spiroplasma symbionts of Myrmica.
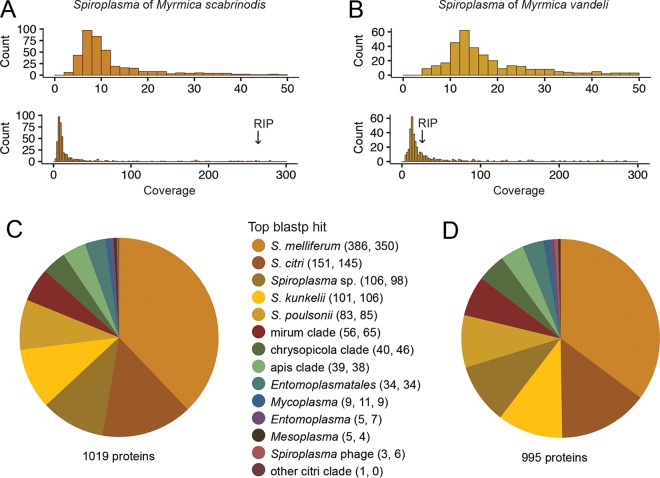
Spiroplasma genomes were sequenced from two host species, M. vandeli and M. scabrinodis. Eighty-seven and eighty-six million reads, respectively, were generated from the pooled DNA of five ants per species. Preliminary metagenomes were assembled from low-GC reads (≤31%) and consisted almost exclusively of ant, Wolbachia, and Spiroplasma contigs by blastp (Table 2). These preliminary Spiroplasma contigs were used to improve the mapping and assembly of Spiroplasma reads in each final assembly (see Materials and Methods). From the final assemblies, 481 contigs encoding 1,019 proteins and 402 contigs encoding 995 proteins were assigned to the Spiroplasma symbionts of M. scabrinodis and M. vandeli, respectively. Of these, 98.4% of M. scabrinodis proteins were also identified in M. vandeli, and 96.8% in the reciprocal comparison, suggesting that the majority of protein-coding genes are represented in our Spiroplasma assemblies. As expected, the vast majority of these putative genes also yielded blastp hits to the genomes of S. citri, S. kunkelii, S. melliferum, and S. poulsonii (Table 2). The respective genome read coverages for M. scabrinodis and M. vandeli were 10.4 and 17.2 (median coverage) and 9.1 and 13.6 (mode coverage) (Fig. 3A and B). A majority fraction of the top blastp hits for each Spiroplasma assembly was to taxa belonging to the citri clade; 70.8% of 1,019 genes in M. scabrinodis and 68.9% of 995 genes in M. vandeli (Fig. 3C and D). The species receiving the largest fraction of top hits was Spiroplasma melliferum, a honeybee pathogen closely allied with the plant pathogens S. citri and S. kunkelii. Genome sequencing facilitated a more thorough comparison of nucleotide identity between strains than the ftsZ locus alone; across 30 kb of syntenic coding and intergenic sequences, the two share 95% identity.
TABLE 2.
Genome assembly statistics of novel Spiroplasma genomes
| Genome assembly | No. (%) for: |
|
|---|---|---|
| Myrmica scabrinodis | Myrmica vandeli | |
| Preliminary metagenomes | ||
| Contigs (≥300 nta) | 113,714 | 107,962 |
| N50 | 593 | 605 |
| Wolbachia genes | 338 | 574 |
| Final Spiroplasma genomes | ||
| Contigs (>300 nt) | 481 | 402 |
| N50 | 3,477 | 4,471 |
| nt | 1,150,673 | 1,206,483 |
| ORFs (>300 nt) | 1,019 | 995 |
| With matches to genome of: | ||
| S. citri | 957 (93.9) | 916 (92.1) |
| S. kunkelii | 928 (91.1) | 891 (89.5) |
| S. melliferum | 895 (87.8) | 881 (88.5) |
| S. poulsonii | 936 (91.8) | 907 (91.2) |
nt, nucleotides.
FIG 3.
Genome coverage and Spiroplasma gene assignment of Myrmica symbionts. Read coverage distribution graphed in a 0 to 50 range and 0 to 300 range for M. scabrinodis (A) and M. vandeli (B) Spiroplasma symbionts. Read coverage of the RIP-encoding contig for each symbiont is indicated with arrows. Pie charts summarize gene assignments within each symbiont's genome. The majority fraction of Spiroplasma genes in M. scabrinodis (C) and M. vandeli (D) Spiroplasma symbionts match best by blastp to the honeybee pathogen Spiroplasma melliferum, and overall, most genes match to members of the citri clade: Spiroplasma citri, S. kunkelii, S. melliferum, and S. poulsonii. The respective numbers of top blastp matches from M. scabrinodis and M. vandeli symbionts are indicated in parentheses.
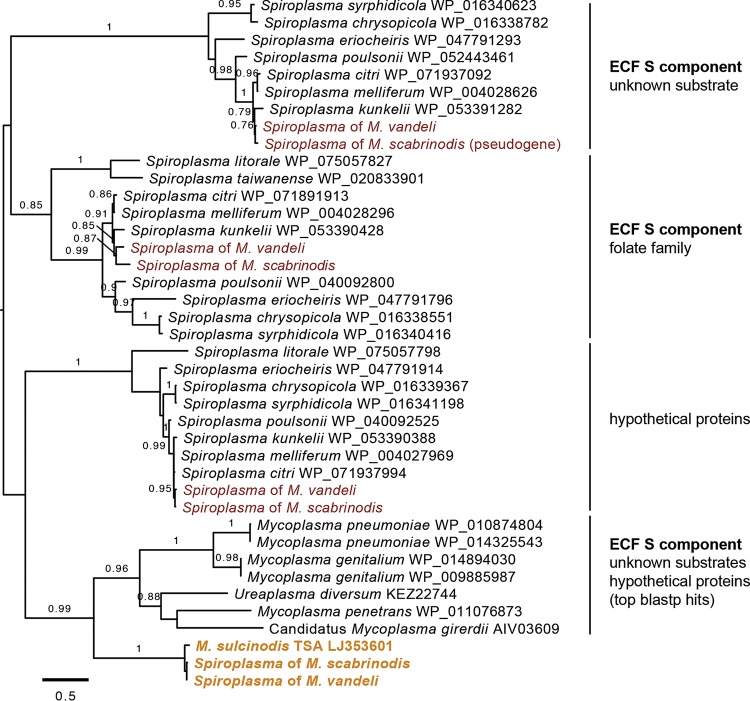
Genes that are unique to these strains relative to other Spiroplasma taxa may hint toward the biological role of Spiroplasma in Myrmica. Hypothetical open reading frames (ORFs) located on the same contig as a Spiroplasma gene were translated and queried by blastp and HMMER against the nr protein database and reference proteomes. Using a conservative minimum of 600 nucleotides for ORF prediction, nine and eight candidates from M. scabrinodis and M. vandeli, respectively, were identified. All but one returned no significant similarity or domain conservation to known proteins. The exception encodes a nutrient transporter gene that is absent from all other sequenced Spiroplasma genomes: the substrate component of an energy-coupling factor (ECF) membrane transporter. No disruption in read coverage was evident between the ECF transporter and the Spiroplasma genes flanking it; it uses the Mycoplasma/Spiroplasma genetic code and is also present in the transcriptome of M. sulcinodis, suggesting it is not an artifact of contaminating sequence reads in the assembly. PCR screens confirmed its presence in M. vandeli and both M. scabrinodis strains, but did not yield amplicons from Myrmica specimens from outside the scabrinodis species group. A phylogenetic analysis alongside the most similar blastp matches and ECF transporter gene families of other Spiroplasma taxa placed the putative novel transporter on a long branch, distantly related to characterized families (Fig. 4).
FIG 4.
Divergent ECF transporters in the genomes of Myrmica Spiroplasma symbionts. Shown is a maximum-likelihood phylogram of energy-coupling factor (ECF) transporter substrate component amino acid sequences. Tips are labeled with taxonomic identifiers and clades with protein family information, if available. Gold type indicates the novel ECF transporter of Myrmica-associated Spiroplasma symbionts, and red type indicates other ECF transporters identified in the genomes of these symbionts. Branches are labeled with SH-like approximate likelihood ratio test scores of 0.75 or higher.
Ribosome-inactivating proteins in Myrmica Spiroplasma symbionts.
Ribosome-inactivating protein (RIP)-coding regions were identified in each of the Spiroplasma genomes and in the transcriptome of M. rubra. The RIP-coding region of M. vandeli is not predicted to encode a functional protein due to reading frame disruptions, while those of M. scabrinodis and M. rubra appear to encode intact ORFs with conserved active site residues, though the latter is only partially represented (∼60% of the gene). Phylogenies of RIPs are not congruent with hosts (Fig. 5), as was found in other Spiroplasma strains (29). The RIP of M. scabrinodis assembled into a 14-kb contig with an order of magnitude greater read coverage than that of M. vandeli, suggesting copy number variation between the two (Fig. 3A and B). The products of other genes on this contig display strong amino acid sequence similarity to Spiroplasma proteins involved in type IV secretion systems used for conjugative DNA transfer, such as the proteins encoded by soj, mob, and traE (35, 36).
FIG 5.
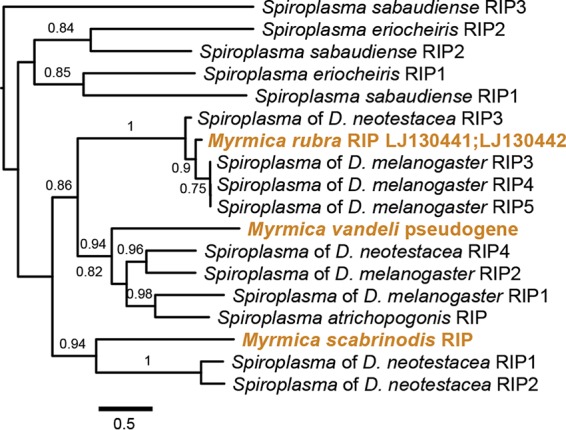
Diversity of ribosome-inactivating proteins in Myrmica Spiroplasma. Shown is a maximum-likelihood phylogram of Spiroplasma-encoded ribosome inactivating protein (RIP) amino acid sequences. Tips are labeled with Spiroplasma species names or references to the host species harboring a Spiroplasma symbiont. Gold type indicates a RIP sequence identified from a Myrmica-associated Spiroplasma symbiont.
The presence of RIPs was confirmed by PCR for both M. vandeli and M. scabrinodis, while reactions targeting the M. rubra RIP failed to amplify from our North American samples. The detection of RIPs from individual colonies of scabrinodis group hosts varied by Spiroplasma strain. The RIP pseudogene was detected in workers from each of the two colonies of M. vandeli, while the intact RIP was detected in all of the colonies of M. scabrinodis bearing Spiroplasma haplotype B but none of the haplotype A colonies.
DISCUSSION
In this study, Myrmica ants are shown to be a hot spot for Spiroplasma infection. Eight of nine species screened in the present study, as well as all three species with publicly available transcriptomes, harbor Spiroplasma. In addition, the infection prevalence within species is high, with 42 of 43 and 250 of 276 colonies and individuals, respectively, infected. No strains were shared by multiple ant species, while one species, M. ruginodis, hosted two different strains. Other broad surveys of symbionts in ants, using universal 16S rRNA primers, have also reported Spiroplasma infections from multiple species groups, including citri, ixodetis, and platyhelix (see Fig. S1 in the supplemental material), in 27 of 95 species (37) and in 24 of 464 species (38). In the latter study, one half of infections were in the genus Polyrhachis. Although these screens do not typically distinguish between inherited and horizontally transmitted Spiroplasma strains or provide information about prevalence within ant species, they suggest that Spiroplasma infections may not be uncommon in ants.
The perfect association between mitochondrial haplotype and Spiroplasma variant, which was found in our detailed screening of microsympatric colonies of M. scabrinodis and M. vandeli, is strong evidence for symbiont vertical transmission. Two mitochondrial haplotypes were found in M. scabrinodis (96% similar at COI), and these are perfectly associated with Spiroplasma haplotypes, as indicated by variation in the ftsZ gene (99% similar). Individuals (and colonies) with different mitochondrial haplotypes showed no differences in their nuclear genes, and it is not known how or why this mitochondrial polymorphism persists. This pattern of genetic variation is in contrast with that in M. rubra, where nuclear but not mitochondrial differences are associated with a queen reproductive polymorphism (39, 40). The close relative M. vandeli harbors a closely related Spiroplasma strain (95% similar to M. scabrinodis symbionts, 99% at ftsZ alone). It is interesting that these ants do not share or appear to exchange Spiroplasma strains despite being found so close to each other, and it is in contrast to other studies finding the exchange of Wolbachia between socially parasitic ants and those within the host colonies (41–43). Interestingly, one of these studies found that unlike Wolbachia, Spiroplasma strains were not exchanged between the host and the social parasite (43). Finally, the high infection prevalence in Myrmica larvae and pupae and widespread tissue distribution also suggest vertical transmission.
All Spiroplasma strains in the present study were from the citri clade. A 16S rRNA screen also found a strain from the citri clade infecting Myrmica incompleta (38). The Myrmica symbionts are not monophyletic; plant pathogens S. kunkelii and S. citri, bee pathogenic S. melliferum, and symbionts of Drosophila wheeleri, D. aldrichi, and D. mojavensis are all nested within the group of Myrmica Spiroplasma. Although there is not strict cospeciation between Myrmica ants and their symbionts, there is a strong phylogenetic signal, with three lineages of Spiroplasma each closely associated with a lineage of Myrmica; although, much more detailed sampling and screening of Myrmica is required to determine how many independent acquisitions of Spiroplasma have occurred. This will be challenging, as Myrmica is a very diverse clade of ants whose taxonomy and evolutionary relationships are unresolved and sometimes controversial, with many cryptic species (39, 44–46). We know of no cases of cospeciation between Spiroplasma strains with their hosts, and in general, cospeciation between facultative symbionts and their hosts is very rare (see reference 47 for a Wolbachia example). Most facultative symbionts are lost before their hosts speciate and infect new hosts via horizontal transmission (30, 48, 49).
The patterns of association between Spiroplasma and Myrmica differ greatly from that with Drosophila, the best-studied insect lineage that is commonly infected by inherited Spiroplasma. There, at least five lineages from four clades have colonized Drosophila, and hosts are often infected at low frequencies, largely depending on maternal transmission efficiency, as well as the fitness and phenotypic effects of the symbiont. For example, male-killing strains are found at low frequencies in host populations (50), whereas a strain that protects against a very common virulent nematode parasite occurs at a high frequency (33).
Of course, the obvious next step is to determine what effects Spiroplasma strains might have on their Myrmica hosts. It is unlikely that these microbes are essential, as some individuals (and one species) were uninfected. Obligate inherited symbionts of ants include strains of “Candidatus Blochmannia,” which recycle nitrogen for their carpenter ant hosts (51); however, Myrmica ants are primarily predaceous and not thought to feed on nutrient-limited diets (52). Also, no Spiroplasma strains are known to be obligate symbionts.
Perhaps Spiroplasma manipulates Myrmica reproduction, for example, by killing males. It is challenging though to demonstrate sex ratio distortion in ants and other social Hymenoptera, as this would involve isolating symbiont-free queens, rearing colonies to produce reproducers, and comparing their sex ratios with those of infected colonies. As far as we are aware, only one study has shown a convincing link between symbionts and sex ratio distortion in ants (53). In that study, artificial selection on the sex ratios in colonies of the pharaoh ant Monomorium pharaonis resulted in rapid changes in the frequency of Wolbachia infections.
Another possibility is that Spiroplasma strains persist in Myrmica by providing protection against natural enemies. Myrmica ants are commonly infected with parasites (54). In fact, new species of Myrmica have been erroneously described due to parasitic nematodes, because infected ants often look different from uninfected ones, with distended abdomens (55, 56). To explore the potential for protection, we sequenced Spiroplasma genomes and surveyed for ribosome-inactivating proteins (RIPs), toxins that are widespread and diverse in Spiroplasma and that have been implicated in defense against parasitic nematodes and wasps (28, 29). These toxins appear to evolve rapidly and exhibit elevated rates of gains and losses in Spiroplasma, making the initial detection by PCR difficult, even with degenerate primers. However, our genome surveys uncovered two RIPs. One of these, that associated with M. vandeli, is a pseudogene, while a plasmid associated with M. scabrinodis haplotype B (but not A) encodes the other. That the RIP toxins are pseudogenized or found on plasmids suggests that their host associations are dynamic, perhaps evolving in concert with changing pressures from natural enemies. In support of this, we also uncovered a Spiroplasma RIP from the transcriptome of M. rubra collected from its native range in Europe, but we could not detect it in North American colonies, suggesting that it also occurs on a plasmid and was lost, perhaps due to enemy release, when M. rubra invaded North America. Of course, much work remains to determine whether these RIPs might be protective and against what.
Genomes can also provide useful clues for understanding symbiont biology (1). We searched the Myrmica-Spiroplasma metagenome for novel Spiroplasma genes, i.e., genes that do not occur in any sequenced Spiroplasma genomes, of which 21 are currently available from four clades, including S. melliferum, S. citri, and S. kunkelii from the citri clade. All but one of the new genes were of unknown function, and there were no new metabolic pathways uncovered, further suggesting that the symbiont does not fill an obligate nutritional or metabolic role for its host. We identified a divergent ECF substrate (S) component gene responsible for conferring substrate specificity to a transport complex. In shared energy-coupling transport systems, an ATPase binding cassette and transmembrane protein, the so-called AT module, are universal components of each transporter, with substrate specificity being conferred by S component genes (57). Substrates include vitamins and transition metals; therefore, it is possible that Spiroplasma symbionts of Myrmica are supplementing or siphoning nutrients. Phylogenetic analysis can help to identify candidate protein functions among members of specialized protein classes. However, this gene could not be conclusively attributed to a characterized substrate family; thus, its role and importance in this symbiosis remain open questions.
Finding such prevalent inherited Spiroplasma in Myrmica opens up many interesting questions. The next step is to perform experiments comparing symbiont-infected and uninfected ants after antibiotic treatment of lab colonies. A promising model would be the European fire ant Myrmica rubra, one of the most invasive ant species globally. Interestingly, a little-cited study from 20 years ago treated M. rubra lab colonies with antibiotics and found an effect on ant growth and queen production (58).
MATERIALS AND METHODS
Sample collection, DNA extraction, and Spiroplasma screening.
Individuals from 29 ant colonies from two sites that were 35 m apart (site 1, 46.7594°N, 6.2527°E; and site 2, 46.7595°N, 6.2573°E) in the Natural Reserve of Lac de Remoray, France, were collected in August 2016. This area was already known to harbor a number of different Myrmica species. Species determinations were made by Mesut Koken, a local ant expert. The number of ants sampled from each colony ranged from 6 to 20; for one M. scabrinodis colony, seven larvae and 10 pupae were also collected. Colony samples were stored separately in 95% ethanol. In addition, Myrmica samples were received from colleagues as whole ants in ethanol or as DNA extractions (see Acknowledgments and Table S1 in the supplemental material).
In preparation for DNA extraction, ants were removed from ethanol and air dried for 10 min. DNA was extracted from individual ants using the PrepMan Ultra (Applied Biosystems) sample preparation reagent. To rule out the possibility that Spiroplasma was an ectosymbiont harbored on the ant cuticle, all sampled individuals from one M. scabrinodis colony were surface sterilized by submerging ant specimens in 2.5% bleach for 2 min and then in 70% ethanol for 4 min and then rinsing them in distilled water twice for 3 min. To test for systemic infection, the heads, thoraces, gasters, and legs of eight ants from one M. scabrinodis and one M. vandeli colony were carefully dissected, and DNA was extracted and screened separately.
DNA extractions were screened for Spiroplasma by PCR using primers targeting a 780-bp fragment of the single-copy cell division protein gene ftsZ (F2, 5′-TGAACAAGTCGCGTCAATAAA; and R2, 5′-CCACCAGTAACATTAATAATAGCATCA [30]). Some initial screens were also performed using primers targeting 300 bp of Spiroplasma 16S rRNA (Spi16S-F, 5′-CCTGAGTAGTATGCTCGCAAGAG; and Spi16S-R, 5′-CCCACCTTCCTCTAGCTTAC). Primers targeting the Myrmica host gene, mitochondrially encoded cytochrome oxidase subunit I (COI), were used as a positive control for DNA quality and to sequence a region of Myrmica DNA for molecular identification and analysis. The primers and sequences are MyrmCOI F, 5′-TCGTTTAGAATTAGGATCTTGT; and MyrmCOI R, 5′-ATGAGAAATTAATCCAAATCCAG for species in the scabrinodis group taxa; uMyrmicaCOI F, 5′-TAATTAATAATGAYCAAATTTATAATAC; and uMyrmicaCOI R, 5′-GTRGGRATTGCAATAATTATAGTTGC for all other Myrmica taxa; and LCO1490, 5′-TAAACTTCAGGGTGACCAAAAAATCA; and HCO2198, 5′-GGTCAACAAATCATAAAGATATTGG (59) for Formica. Primers targeting 500 bp of a variable portion of the nuclear-encoded long-wavelength rhodopsin gene (LR143F, 5′-CACTGGTATCARTTCGCACCSAT; and LR672R, 5′-CCRCAMGCWGTCATGTTRCCTTC [46]) were used to confirm that divergent M. scabrinodis mitochondrial haplotypes corresponded to the same species. RIP primers were scabRIP F, 5′-GAGGAACTAAAATTGAAGTAGTTCT; scabRIP R, 5′-AATCTTCATCTTGATACTTGACCAC; vanRIP F, 5′-TCCTTGGTTAGATACTATTTCTGCTC; and vanRIP R, 5′-ATTATTGAGTTTGAGGTATCGC. ECF transporter primers were Sp-ECF F, 5′-CTTAGCAGCTGTAATGTTAGCATTAAC; and Sp-ECF R, 5′-CTAATTCCACAGCCATAAATAAAGTAG. Thermal cycling programs for ftsZ, MyrmCOI, Spi16S, HCO/LCO, LR, RIPs, and ECF primers were 35 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 1 min 15 s, and for uMyrmicaCOI, 35 cycles of 95°C for 30s, 49°C for 30 s, and 72°C for 1 min 15 s. PCR products were assessed by DNA gel electrophoresis on a 1% agarose gel stained with ethidium bromide and visualized under UV light. One or more ftsZ and COI amplicons per Myrmica species was sequenced with the Sanger method by Sequetech (CA, USA) using forward and reverse primers. At least one ftsZ amplicon for each host taxon in our sample set was sequenced. Intrahost symbiont diversity was examined by sequencing ftsZ and COI from all individuals from one M. vandeli and two M. scabrinodis colonies (n = 10, 9, and 8 individuals, respectively).
Sequence processing and phylogenetic analysis.
Primer regions were trimmed from the Sanger sequences by hand, yielding final products of 648 bases for ftsZ and 690 bases for COI. ftsZ sequences were used to query nucleotide sequences deposited in the NCBI transcriptome shotgun assembly (TSA) database. For Myrmica taxa with TSA hits to Spiroplasma ftsZ sequences, COI sequences were also recovered from the transcriptome by blastn. Nucleotide and amino acid sequences were aligned with MAFFT 7.309 (60). For each nucleotide alignment, the best substitution model for phylogenetic analysis was determined in jModelTest 2.1.7 (61). For ftsZ, the best model was HKY+I+G and for COI it was TPM2uf+G. RIP and ECF transporter phylogenies were built from amino acid alignments using the LG substitution model. Maximum-likelihood phylograms were constructed using PhyML 2.2.0 (62) implemented in Geneious R10. SH-like approximate likelihood ratio test scores were calculated for each branch. Alignments and phylogenies used to test for evidence of cophylogeny were generated as described above. Tests of codivergence were carried out with ParaFit (63) implemented in the R package ape (64). Briefly, patristic distance matrices were calculated for the host COI and symbiont ftsZ loci using the same models of nucleotide substitution as above. Included in these alignments were all the hosts that were positive for Spiroplasma by our PCR screens, 10 distinct host mitochondrial lineages and their symbionts, plus M. ruginodis host and Spiroplasma sequences from the public transcriptome. Distance matrices were permuted randomly to create a distribution of data against which to test the null hypothesis of independent host and symbiont evolutionary histories. Matrices were permuted 999 times. Because infection frequency in M. ruginodis could not be determined nor could read contamination in the sequence read archive be ruled out, the codivergence analysis was carried out once with and once without this host. The tanglegram depicted in Fig. 2 was visualized with TreeMap 3 (65).
Spiroplasma genome DNA extractions, sequencing, and assembly.
To prepare samples for Illumina sequencing, genomic DNA was extracted from pools of five ants for each of two species in the scabrinodis clade, Myrmica scabrinodis haplotype B and M. vandeli, using the phenol-chloroform method. Short-insert shotgun libraries were prepared and 125-bp paired-end reads were sequenced by Genome Québec (Montréal, Québec, Canada) on a HiSeq 2500 v4 system.
Preliminary metagenomes were assembled for each of the two Myrmica host species, M. vandeli and M. scabrinodis, after filtering out reads with GC contents greater than 31% and their pairs. The low-GC data set was mapped to the mitochondrial genome sequence of Myrmica scabrinodis (NCBI reference sequence NC_026133) and removed. Reads were trimmed, filtered, and mapped with BBMap 37.36 (by Brian Bushnell, https://sourceforge.net/projects/bbmap/). Filtered reads were assembled de novo using SPAdes 3.10.1 (66). Open reading frames with a minimum length of 300 nucleotides were predicted and translated with Geneious R10 and compared against the nr protein database available on NCBI using blastp. All contigs encoding Spiroplasma genes, i.e., sequences for which the top blastp hit was to Spiroplasma, were retained as a preliminary genome assembly. Spiroplasma genes with higher GC contents, though rare, were absent from these assemblies. To produce a more complete assembly for each symbiont strain, a second iteration of mapping and assembly was carried out. Using the original read sets, reads were quality trimmed and GC filtered at 45%. All remaining reads that mapped to contigs in the preliminary assemblies, as well as to the sequenced genome of Spiroplasma citri and its plasmids, were again assembled de novo using SPAdes. The blastp search was repeated to identify Myrmica and bacterial contigs that had not been removed through the mapping procedure. A collection of genes with top hits to either non-Spiroplasma or unclassified Entomoplasmatales taxa, 56 in M. vandeli and 53 in M. scabrinodis, were interpreted as Spiroplasma genes nonetheless if they were located on Spiroplasma contigs and showed strong blastp hits to Spiroplasma taxa as well.
To identify genes unique to the Myrmica Spiroplasma, proteins with top blastp matches to Spiroplasma were annotated back onto the assembly contigs and compared against all predicted ORFs on each contig, i.e., most ORFs had two annotations, one as a predicted protein-coding gene and one as a Spiroplasma blastp match. ORFs with only the former annotation were investigated individually with blastp, blastn, and HMMER to identify putative functions. RIPs were identified by tblastn to the final assemblies, to the three Myrmica transcriptomes, and to sequence read archives available as part of NCBI BioProject number PRJDB4088.
Data availability.
Genomic DNA sequence reads and PCR amplicon sequences generated during this study (67) have been submitted to GenBank under BioProject number PRJNA419549 and accession numbers MG558353 to MG558456.
Supplementary Material
ACKNOWLEDGMENTS
We thank Megan Frederickson, Rob Higgins, Danielle Hoefele, Caroline Cameron, Corrie Moreau, and Jake Russell for donating Myrmica specimens and Mesut Koken for assisting in collecting and identifying specimens. We thank the staff at Réserve Naturelle Nationale du Lac de Remoray, in particular Jocelyn Claude, for hospitality and field assistance. We also thank Ryan Gawryluk and Cuong Le for technical advice.
This work was funded by a Sinergia grant from the Swiss National Science Foundation awarded to S.J.P. and a Jamie Cassels Undergraduate Research Award from the University of Victoria to L.D.M.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02299-17.
REFERENCES
- 1.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 2.Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver KM, Russell JA, Moran NA, Hunter MS. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci U S A 100:1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie J, Vilchez I, Mateos M. 2010. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS One 5:e12149. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellner RLL, Dettner K. 1996. Differential efficacy of toxic pederin in deterring potential arthropod predators of Paederus (Coleoptera: Staphylinidae) offspring. Oecologia 107:293–300. doi: 10.1007/BF00328445. [DOI] [PubMed] [Google Scholar]
- 6.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira L, Ferreira Á, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. 2010. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 9.Hurst GDD, Jiggins FM, Hinrich Graf von der Schulenburg J, Bertrand D, West SA, Goriacheva II, Zakharov IA, Werren JH, Stouthamer R, Majerus MEN. 1999. Male-killing Wolbachia in two species of insect. Proc R Soc Lond B Biol Sci 266:735–740. doi: 10.1098/rspb.1999.0698. [DOI] [Google Scholar]
- 10.Jiggins FM, Hurst GD, Jiggins CD, von der Schulenburg JH, Majerus ME. 2000. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology 120:439–446. doi: 10.1017/S0031182099005867. [DOI] [PubMed] [Google Scholar]
- 11.Majerus TMO, Graf von der Schulenburg JH, Majerus MEN, Hurst GDD. 1999. Molecular identification of a male-killing agent in the ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Insect Mol Biol 8:551–555. doi: 10.1046/j.1365-2583.1999.00151.x. [DOI] [PubMed] [Google Scholar]
- 12.Stouthamer R, Breeuwert JA, Luck RF, Werren JH. 1993. Molecular identification of microorganisms associated with parthenogenesis. Nature 361:66–68. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- 13.Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M. 1992. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc R Soc Lond B Biol Sci 250:91–98. doi: 10.1098/rspb.1992.0135. [DOI] [PubMed] [Google Scholar]
- 14.Duron O, Bouchon D, Boutin SSS, Bellamy L, Zhou L, Engelstadter J, Hurst GD, Engelstädter J. 2008. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlman SJ, Hunter MS, Zchori-Fein E. 2006. The emerging diversity of Rickettsia. Proc R Soc Lond B Biol Sci 273:2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasparich GE, Whitcomb RF, Dodge D, French FE, Glass J, Williamson DL. 2004. The genus Spiroplasma and its non-helical descendants: phylogenetic classification, correlation with phenotype and roots of the Mycoplasma mycoides clade. Int J Syst Evol Microbiol 54(Pt 3):893–918. doi: 10.1099/ijs.0.02688-0. [DOI] [PubMed] [Google Scholar]
- 17.Ding Z, Tang J, Xue H, Li J, Ren Q, Gu W, Meng Q, Wang W. 2014. Quantitative detection and proliferation dynamics of a novel Spiroplasma eriocheiris pathogen in the freshwater crayfish, Procambarus clarkii. J Invertebr Pathol 115:51–54. doi: 10.1016/j.jip.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Clark TB, Whitcomb RF, Tully JG, Mouches C, Saillard C, Bove JM, Wroblewski H, Carle P, Rose DL, Henegar RB, Williamson DL. 1985. Spiroplasma melliferum, a new species from the honeybee (Apis mellifera). Int J Syst Bacteriol 35:296–308. doi: 10.1099/00207713-35-3-296. [DOI] [Google Scholar]
- 19.Whitcomb RF, Chen TA, Williamson DL, Liao C, Tully JG, Bové JM, Mouches C, Rose DL, Coan ME, Clark TB. 1986. Spiroplasma kunkelii sp. nov.: characterization of the etiological agent of corn stunt disease. Int J Syst Evol Microbiol 36:170–178. doi: 10.1099/00207713-36-2-170. [DOI] [Google Scholar]
- 20.Saglio P, Lhospital M, Lafleche D, Dupont G, Bove JM, Tully JG, Freundt EA. 1973. Spiroplasma citri gen. and sp. n.: a mycoplasma-like organism associated with “stubborn” disease of citrus. Int J Syst Bacteriol 23:191–204. doi: 10.1099/00207713-23-3-191. [DOI] [Google Scholar]
- 21.Hackett KJ, Whitcomb RF, French FE, Tully JG, Gasparich GE, Rose DL, Carle P, Bové JM, Henegar RB, Clark TB, Konai M, Clark EA, Williamson DL. 1996. Spiroplasma corruscae sp. nov., from a firefly beetle (Coleoptera: Lampyridae) and tabanid flies (Diptera: Tabanidae). Int J Syst Bacteriol 46:947–950. doi: 10.1099/00207713-46-4-947. [DOI] [PubMed] [Google Scholar]
- 22.Whitcomb RF, French FE, Joseph G, Gasparich GE, Rose DL, Carle P, Bove JM, Henegar RB, Konai M, Hackett KJ, Adams JR, Williamson DL, Truman B. 1997. Spiroplasma chrysopicola sp. nov., Spiroplasma gladiatoris sp. nov., Spiroplasma helicoides sp. nov., and Spiroplasma tabanidicola sp. nov., from Tabanid (Diptera: Tabanidae) flies. Int J Syst Bacteriol 47:713–719. [Google Scholar]
- 23.Sakaguchi B, Poulson DF. 1961. Distribution of “sex-ratio” agent in tissues of Drosophila willistoni. Genetics 46:1665–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi M, Watanabe M, Yukuhiro F, Nomura M, Kageyama D. 2016. A nightmare for males? A maternally transmitted male-killing bacterium and strong female bias in a green lacewing population. PLoS One 11:e0155794. doi: 10.1371/journal.pone.0155794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanada-Morimura S, Matsumura M, Noda H. 2013. Male killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus. J Hered 104:821–829. doi: 10.1093/jhered/est052. [DOI] [PubMed] [Google Scholar]
- 26.Poulson DF, Sakaguchi B. 1961. Nature of “sex-ratio” agent in Drosophila. Science 133:1489–1490. doi: 10.1126/science.133.3463.1489. [DOI] [PubMed] [Google Scholar]
- 27.Łukasik P, van Asch M, Guo H, Ferrari J, Godfray HC. 2013. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16:214–218. doi: 10.1111/ele.12031. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton PT, Peng F, Boulanger MJ, Perlman SJ. 2016. A ribosome-inactivating protein in a Drosophila defensive symbiont. Proc Natl Acad Sci U S A 113:350–355. doi: 10.1073/pnas.1518648113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballinger MJ, Perlman SJ. 2017. Generality of toxins in defensive symbiosis: ribosome-inactivating proteins and defense against parasitic wasps in Drosophila. PLoS Pathog 13:e1006431. doi: 10.1371/journal.ppat.1006431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haselkorn TS, Markow TA, Moran NA. 2009. Multiple introductions of the Spiroplasma bacterial endosymbiont into Drosophila. Mol Ecol 18:1294–1305. doi: 10.1111/j.1365-294X.2009.04085.x. [DOI] [PubMed] [Google Scholar]
- 31.Chandler JA, Lang J, Bhatnagar S, Eisen JA, Kopp A. 2011. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet 7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watts T, Haselkorn TS, Moran NA, Markow TA. 2009. Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS One 4:e5703. doi: 10.1371/journal.pone.0005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cockburn SN, Haselkorn TS, Hamilton PT, Landzberg E, Jaenike J, Perlman SJ. 2013. Dynamics of the continent-wide spread of a Drosophila defensive symbiont. Ecol Lett 16:609–616. doi: 10.1111/ele.12087. [DOI] [PubMed] [Google Scholar]
- 34.Seifert B, Yazdi AB, Schultz R. 2014. Myrmica martini sp.n.–a cryptic species of the Myrmica scabrinodis species complex (Hymenoptera: Formicidae) revealed by geometric morphometrics and nest-centroid clustering. Myrmecol News 19:171–183. [Google Scholar]
- 35.Bai X, Fazzolari T, Hogenhout SA. 2004. Identification and characterization of traE genes of Spiroplasma kunkelii. Gene 336:81–91. doi: 10.1016/j.gene.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Joshi BD, Berg M, Rogers J, Fletcher J, Melcher U. 2005. Sequence comparisons of plasmids pBJS-O of Spiroplasma citri and pSKU146 of S. kunkelii: implications for plasmid evolution. BMC Genomics 6:175. doi: 10.1186/1471-2164-6-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kautz S, Rubin BER, Moreau CS. 2013. Bacterial infections across the ants: frequency and prevalence of Wolbachia, Spiroplasma, and Asaia. Psyche (Camb Mass) 2013:936341. doi: 10.1155/2013/936341. [DOI] [Google Scholar]
- 38.Russell JA, Funaro CF, Giraldo YM, Goldman-Huertas B, Suh D, Kronauer DJC, Moreau CS, Pierce NE. 2012. A veritable menagerie of heritable bacteria from ants, butterflies, and beyond: broad molecular surveys and a systematic review. PLoS One 7:e51027. doi: 10.1371/journal.pone.0051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner FM, Schlick-Steiner BC, Konrad H, Moder K, Christian E, Seifert B, Crozier RH, Stauffer C, Buschinger A. 2006. No sympatric speciation here: multiple data sources show that the ant Myrmica microrubra is not a separate species but an alternate reproductive morph of Myrmica rubra. J Evol Biol 19:777–787. doi: 10.1111/j.1420-9101.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- 40.Leppänen J, Seppä P, Vepsäläinen K, Savolainen R. 2015. Genetic divergence between the sympatric queen morphs of the ant Myrmica rubra. Mol Ecol 24:2463–2476. doi: 10.1111/mec.13170. [DOI] [PubMed] [Google Scholar]
- 41.Dedeine F, Ahrens M, Calcaterra L, Shoemaker DD. 2005. Social parasitism in fire ants (Solenopsis spp.): a potential mechanism for interspecies transfer of Wolbachia. Mol Ecol 14:1543–1548. doi: 10.1111/j.1365-294X.2005.02499.x. [DOI] [PubMed] [Google Scholar]
- 42.Liberti J, Sapountzis P, Hansen LH, Sørensen SJ, Adams RMM, Boomsma JJ. 2015. Bacterial symbiont sharing in Megalomyrmex social parasites and their fungus-growing ant hosts. Mol Ecol 24:3151–3169. doi: 10.1111/mec.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haapaniemi K, Pamilo P. 2015. Social parasitism and transfer of symbiotic bacteria in ants (Hymenoptera: Formicidae). Myrmecol News 21:49–57. [Google Scholar]
- 44.Radchenko A, Elmes GW. 2003. A taxonomic revision of the socially parasitic Myrmica ants (Hymentoptera: Formicidae) of the Palaearctic region. Annal Zool 53:217–243. [Google Scholar]
- 45.Savolainen R, Vepsalainen K. 2003. Sympatric speciation through intraspecific social parasitism. Proc Natl Acad Sci U S A 100:7169–7174. doi: 10.1073/pnas.1036825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen G, Savolainen R, Vepsäläinen K. 2010. Phylogeny, divergence-time estimation, biogeography and social parasite-host relationships of the Holarctic ant genus Myrmica (Hymenoptera: Formicidae). Mol Phylogenet Evol 56:294–304. doi: 10.1016/j.ympev.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 47.Jaenike J, Stahlhut JK, Boelio LM, Unckless RL. 2010. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol Ecol 19:414–425. doi: 10.1111/j.1365-294X.2009.04448.x. [DOI] [PubMed] [Google Scholar]
- 48.Werren JH, Zhang W, Guo LR. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc Lond B Biol Sci 261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- 49.Jaenike J. 2012. Population genetics of beneficial heritable symbionts. Trends Ecol Evol 27:226–232. doi: 10.1016/j.tree.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Montenegro H, Solferini VN, Klaczko LB, Hurst GDD. 2005. Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol Biol 14:281–287. doi: 10.1111/j.1365-2583.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- 51.Feldhaar H, Straka J, Krischke M, Berthold K, Stoll S, Mueller MJ, Gross R. 2007. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol 5:48. doi: 10.1186/1741-7007-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiedler K, Kuhlmann F, Schlick-Steiner BC, Steiner FM, Gebauer G. 2007. Stable N-isotope signatures of central European ants–assessing positions in a trophic gradient. Insectes Soc 54:393–402. doi: 10.1007/s00040-007-0959-0. [DOI] [Google Scholar]
- 53.Pontieri L, Schmidt AM, Singh R, Pedersen JS, Linksvayer TA. 2017. Artificial selection on ant female caste ratio uncovers a link between female-biased sex ratios and infection by Wolbachia endosymbionts. J Evol Biol 30:225–234. doi: 10.1111/jeb.13012. [DOI] [PubMed] [Google Scholar]
- 54.Witek M, Barbero F, Markó B. 2014. Myrmica ants host highly diverse parasitic communities: from social parasites to microbes. Insectes Soc 61:307–323. doi: 10.1007/s00040-014-0362-6. [DOI] [Google Scholar]
- 55.Czechowski W, Radchenko A, Czechowska W. 2007. Mermithid infestation strikingly alters the morphology of Myrmica rubba (L.) (Hymenoptera: Formicidae): possible taxonomical involvements. Annal Zool 57:325–330. [Google Scholar]
- 56.Csosz S. 2012. Nematode infection as significant source of unjustified taxonomic descriptions in ants (Hymenoptera: Formicidae). Myrmecol News 17:23–31. [Google Scholar]
- 57.Rodionov DA, Hebbeln P, Eudes A, ter Beek J, Rodionova IA, Erkens GB, Slotboom DJ, Gelfand MS, Osterman AL, Hanson AD, Eitinger T. 2009. A novel class of modular transporters for vitamins in prokaryotes. J Bacteriol 191:42–51. doi: 10.1128/JB.01208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearson B, Raybould AF. 1998. The effects of antibiotics on the development of larvae and the possible role of bacterial load in caste determination and diapause in Myrmica rubra (Hymenoptera: Formicidae). Sociobiology 31:77–90. [Google Scholar]
- 59.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299. [PubMed] [Google Scholar]
- 60.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 63.Legendre P, Desdevises Y, Bazin E. 2002. A statistical test for host-parasite coevolution. Syst Biol 51:217–234. doi: 10.1080/10635150252899734. [DOI] [PubMed] [Google Scholar]
- 64.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 65.Charleston MA, Robertson DL. 2002. Preferential host switching by primate lentiviruses can account for phylogenetic similarity with the primate phylogeny. Syst Biol 51:528–535. doi: 10.1080/10635150290069940. [DOI] [PubMed] [Google Scholar]
- 66.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ballinger MJ, Moore LD, Perlman SJ. 2017. Metagenomic sequence reads of Myrmica scabrinodis and Myrmica vandeli. Deposited as a BioProject in GenBank (accession no. PRJNA419549). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genomic DNA sequence reads and PCR amplicon sequences generated during this study (67) have been submitted to GenBank under BioProject number PRJNA419549 and accession numbers MG558353 to MG558456.