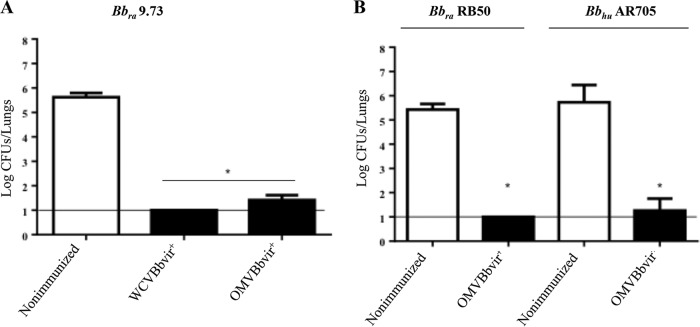
FIG 2.
Effect of i.p. immunization with active OMVBbvir+ in the mouse intranasal challenge model. WCVBbvir+ was used as a positive control. (A) B. bronchiseptica 9.73 (Bbra9.73) was used as the challenge bacteria (5 × 105 CFU, in 40 μl). (B) B. bronchiseptica RB50 (BbraRB50) and the human clinical isolate B. bronchiseptica AR705 (BbhuAR705) were also used as challenge bacteria (5 × 105 CFU, in 40 μl). In all instances, three biological replicates were performed, with the results from a representative one being presented. Results depicted are the means of four mice per group at 7 days after challenge. The horizontal lines indicate the lower limit of detection. In both panels, the numbers of bacteria recovered from the mouse lungs, expressed as the mean ± standard error of the mean (SEM) (error bars) of log10 CFU in the lungs, are plotted on the ordinates for the samples from the lungs of the experimental groups indicated on the abscissas. The Bronchiseptica strains used for the challenges are indicated above the panels. *, significant differences (P < 0.001).