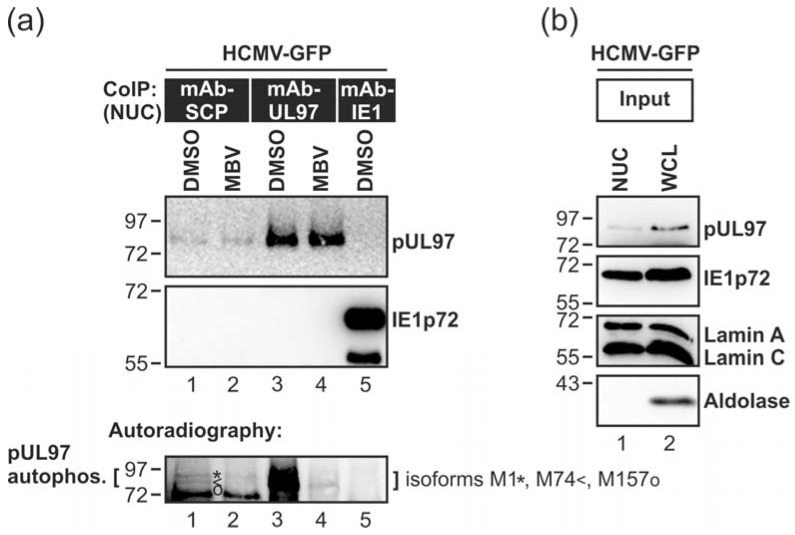
Figure 4.
The kinase activity of capsid-associated pUL97 purified from the nucleus of HCMV-infected cells. HFFs were infected with recombinant HCMV AD169-GFP and treated with the pUL97 kinase inhibitor maribavir (MBV) or the solvent dimethylsulfoxide (DMSO). At 4 dpi, cells were harvested and the nuclear fraction (NUC) was isolated from whole cell lysates (WCL). The nuclear fraction was used for immunoprecipitation of HCMV-encoded proteins SCP, pUL97, or IE1p72 as a control. Precipitates were subjected subsequently to a pUL97-specific in vitro kinase assay (IVKA). ((a), upper panels CoIP) Prior to IVKA reactions, the quality of CoIP samples was verified by Western blot analysis. ((a), lower panel Autoradiography) IVKA reactions were performed under conditions optimized for pUL97 activity and signals of autophosphorylation are depicted. Note the labelling of the three known isoforms of pUL97, M1, M74 and M157, which all possess autophosphorylation activity; (b) The input levels of all relevant proteins were stained on parallel Western blot panels.