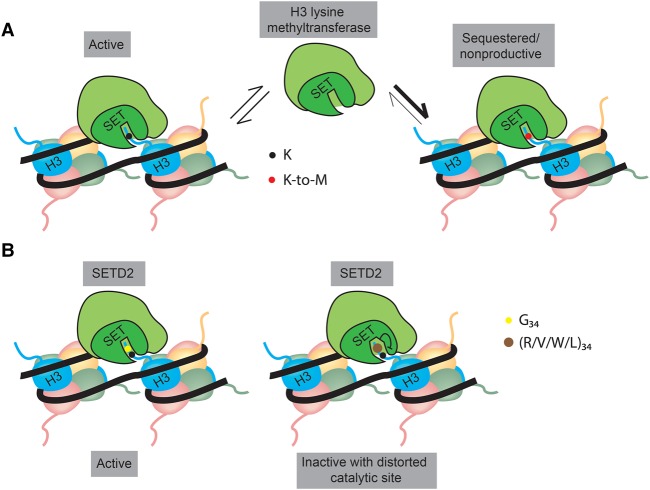
Figure 3.
Proposed mechanisms by which oncohistones affect the activity of histone methyltransferases (HMTs). (A) K-to-M mutant histones bind more tightly to the catalytic site within the SET domain of HMTs than wild-type histones, leading to sequestration of the HMT complex to mutant nucleosomes and rendering the HMT nonproductive due to the absence of the substrate. (B) The G34 residue of histone H3 is buried in a narrow tunnel within the active site of SETD2. Mutation of G34 to residues with a larger side chain (R/V/W/L) might result in distortion of the catalytic site that would render SETD2 inactive.