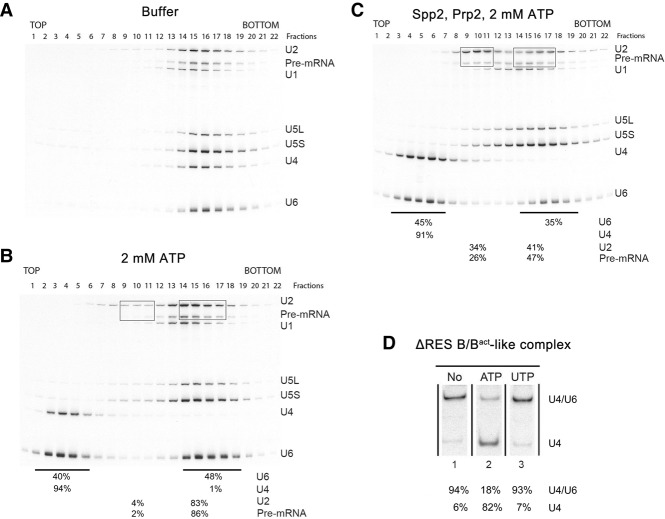
Figure 4.
Affinity-purified ΔRES spliceosomes are partially disassembled upon addition of Prp2, Spp2, and ATP. Spliceosomal complexes formed in extracts from prp2-1 Δsnu17 yeast cells after initial heat inactivation of Prp2 (designated ΔRES B/Bact-like complexes) were affinity-purified, and 45S complexes were subjected to a second glycerol gradient after incubation with buffer (A), 2 mM ATP (B), or ATP, Prp2, and Spp2 (C). RNA was isolated from each gradient fraction, separated by denaturing PAGE, and detected by Northern blotting with 32P-labeled probes against the snRNAs indicated at the right. The 32P-labeled Actin pre-mRNA was detected by autoradiography. The percentage of the pre-mRNA or the indicated snRNAs in the boxed or underlined fractions is shown below the gel in B and C. (D) RNA was isolated from ΔRES B/Bact-like complexes and analyzed by native PAGE. Free U4 snRNA or U4 base-paired with U6 was subsequently visualized by Northern blotting with a 32P probe complementary to U4 snRNA. The percentage of free U4 versus U4 base-paired with U6 was quantitated with a PhosphorImager and is shown below each lane.