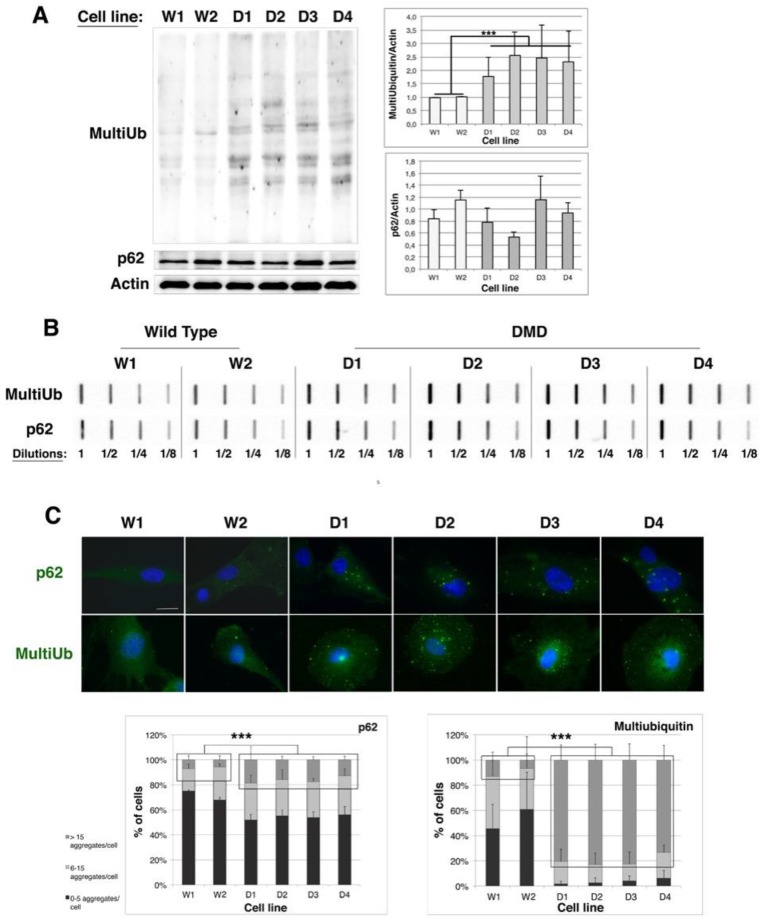
Figure 2.
Protein aggregation is increased in human immortalized DMD myoblast cell lines. (A) 10 µg of total protein extracts of Wild Type (W1 & W2) and DMD (D1, D2, D3 & D4) cell lines were separated by SDS-PAGE and analyzed by immunoblotting, using specific antibodies directed against multi-ubiquitin, p62 or Actin (as a loading control). The histograms show means ± SD of normalized MultiUb/Actin and p62/Actin ratios (MultiUb/Actin or p62/Actin ratio of each cell line was divided by the mean of ratios of control cell lines (W1 and W2)); (n = 3 independent experiments); (B) Filter trap analysis: 2.5 µg of total protein extracts from WT and DMD cell lines were slot-blotted at four different dilutions (1, 1/2, 1/4 & 1/8) on a cellulose acetate membrane and probed with multi-ubiquitin and p62 antibodies. Result is representative of three independent experiments; (C) Immunostaining of multi-ubiquitin and p62 and Hoechst staining of nuclei were performed in Wild Type and DMD myoblasts (scale bar: 50 µm). The graph indicates the percentage of cells containing 0–5, 6–15 and over 15 aggregates (green dots) larger than 2 µm. Counting of aggregates was performed twice on 100 cells per cell line, in three independent experiments. *** p < 0.001, one way ANOVA parametric test.