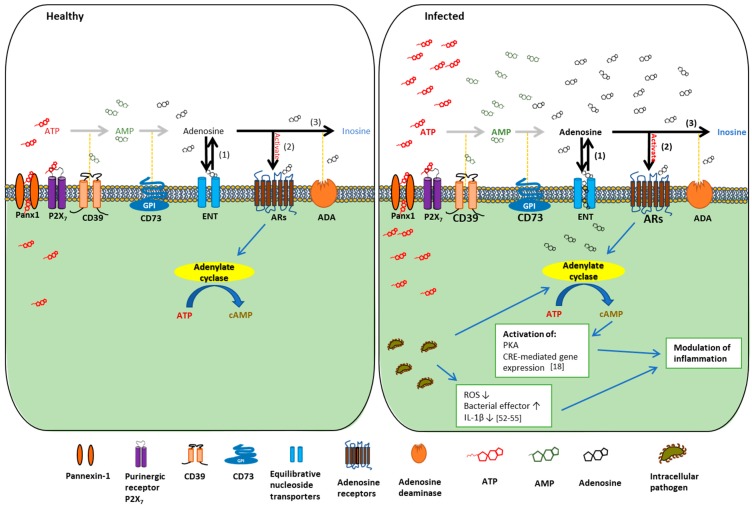
Figure 1.
The change in the metabolism of adenosine signaling between the states of healthy and infected. Adenosine signaling is a crucial contributor to the innate host defense mechanism. Extracellular ATP (eATP) is released to the extracellular space through Pannexin-1 channels (Panx-1). Once in the extracellular space, eATP may activate the P2X7 purinergic receptor or may be converted to adenosine by the CD39/CD73-mediated adenosine pathway. Extracellular adenosine can be metabolized into three separate ways: (1) be transported back into the cell via bi-directional equilibrative nucleoside transporters (ENT) in the direction of concentration gradient; (2) activate adenosine receptors (ARs) on the plasma membrane with the stimulation of P2X7; or (3) be degraded into inosine via adenosine deaminase (ADA). During infection, a substantial increase in the amount of extracellular ATP released by host cells is observed across many species of microbes [23,50,51]. Subsequently, the level of extracellular adenosine and activation of ARs may be altered, potentially changing the outcome of their intracellular signaling cascades. Mucosal pathogens such as P. gingivalis can promote activation of anti-inflammatory ARs. This will further result in elevated cAMP formation via adenylate cyclase activity and subsequent activation of protein kinase A (PKA) and cAMP response element (CRE)-mediated gene expression that are shown to non-redundantly down-regulate inflammation [18]. P. gingivalis has also been shown to attenuate host oxygen-reactive-species (ROS) production and IL-1β release, while increasing bacterial effector secretion [52,53,54,55]. These specific molecular events described above culminate in the modulation of inflammation, which may lead to change the course and severity of infection.