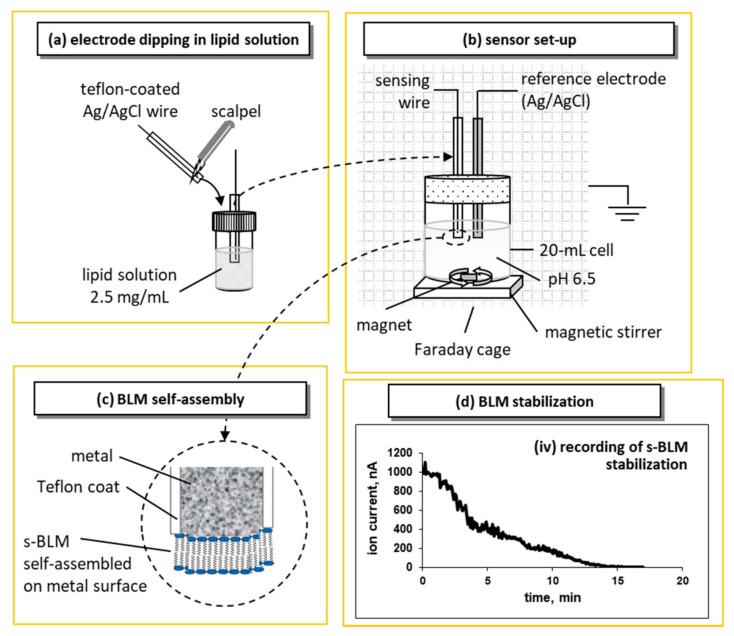
Figure 2.
Schematic of the sensor, measurement setup, and lipid self-assembly process (not drawn to scale): (a) the sensing electrode is tipped with a scalpel and immediately immersed in lipid solution before dipped in the electrolyte solution. (b) The electrochemical setup consists of a 20-mL cell and a two-electrode configuration, i.e., the sensing electrode and a Ag/AgCl reference electrode, placed in a grounded Faraday cage; an external DC potential of 25 mV is applied between the electrodes and the ionic current through the BLM is measured with a digital electrometer; the cell is stirred using a magnetic stirrer. (c) Upon immersion, the lipid droplet attached to the wire is self-assembled into a bilayer that has one layer adsorbed on the metal surface and the other facing the aqueous solution. (d) Recording of the ion current decrease during the self-assembly process; recording started at the immersion of the sensing electrode in the electrolyte solution.