Abstract
Poly(β amino ester) polymers have received growing attention in the literature, owing to their ease of synthesis, versatile co-monomer selection, and highly tunable degradation kinetics. As such, they have shown extensive potential in many biomedical applications as well. In this work, it is demonstrated for the first time that PβAE polymers containing primary and secondary amine groups can undergo degradation by primary alcohols via transesterification mechanism. While this work emphasizes an important aspect of solvent compatibility of these networks, it also represents an interesting, simple mechanism for post synthesis drug incorporation, with riboflavin conjugation being demonstrated as a model compound.
Keywords: Gels, Polymer Drug Conjugate, Drug Delivery, Biomaterials
2. Introduction
Poly(beta-amino ester) (PβAE) gels are hydrolytically degradable systems due to the presence of β-amino ester linkages. As a biodegradable system, this is one of the central features for their applicability in biomedical applications such as gene and drug delivery, and tissue engineering [1–5]. Synthesized via a Michael addition reaction of an acrylate molecule with a primary or secondary amine, the degradation rate of these polymers is considered to be dependent on network structure, chemical composition(e.g., hydrophobicity) and aqueous properties (e.g., pH, temperature) [6–8]. As such they have been shown to degrade faster in the presence of primary and secondary amines in the gel matrix because of the catalyzing action of amines [9, 10]. Additionally, we have previously published a single-step PβAE gel synthesis reaction based upon primary diamine precursors that serve as crosslinkers during polymerization [11]. These networks were found to hydrolyze much more rapidly than gels prepared by a two-step crosslinking system, which is straight chain polymer synthesis followed by radical catalyzed cross-linking of acrylate end-groups [12, 13]. It was theorized that this effect was a result of unmodified amines in the network backbone catalyzing the hydrolysis reaction [14, 15].
Based upon this observation, we hypothesized that these PβAE crosslinked networks would also be sensitive to nucleophilic attack by primary alcohols and the rate of degradation would depend upon the reactivity of alcohols. Herein, we report the first demonstration of PβAE gel degradation by alcohols through a transesterification mechanism, establishing the effect of acrylate to amine ratio, alcohol chain length and degree of carbon substitution on the degradation kinetics. This mechanism also permitted the post synthesis modification of the gels with drug molecules containing alcohol groups, allowing for an easy to use approach for coupling drugs into the backbone of the polymer. the resulting esterified prodrugs can the be activated to release the drug via simple hydrolysis or enzymatic hydrolysis, which is reported as the activation process for 49% of the marketed prodrugs [16, 17]. Hence, this approach could be useful for delivering pharmaceutically active compound having −OH group with unattractive physicochemical properties and would serve as a drug release vehicle in the biophysical environment.
3. Materials
Poly (ethylene glycol) 400 diacrylate (PEG400DA) was purchased from Polysciences Inc., 4, 7, 10-Trioxa-1, 13-tridecane diamine (TTD) and riboflavin were obtained from Sigma-Aldrich (St. Louis, MO). All organic solvents were either purchased from Sigma-Aldrich or Pharmco-aaper (Shelbyville, KY) and were used as received. All the other chemicals were used as received without further purification.
4. Methods
4.1 Poly(β-amino ester) gel film synthesis
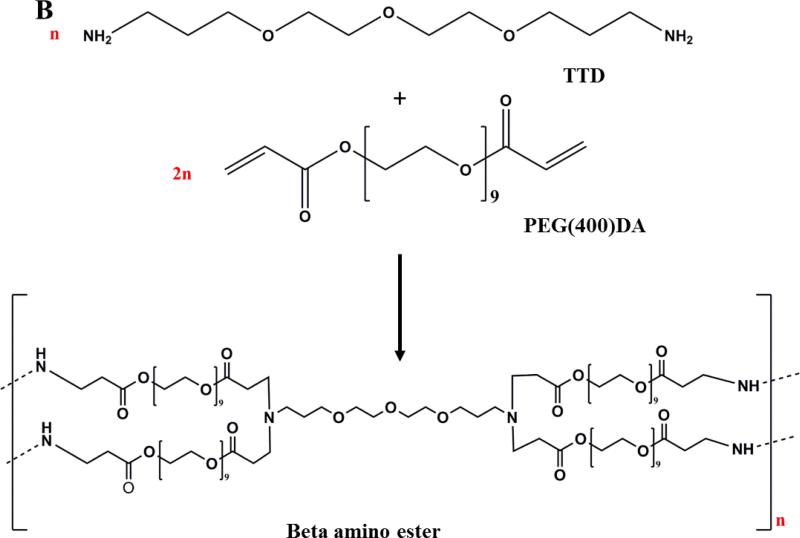
A one-step Michael addition reaction between acrylates and amines was used to synthesize PβAE gels films, as previously described [9, 18]. Figure 1 (A) and 1 (B) depicts the reaction mechanism and the schematic of the predicted crosslinked gel structure. Diacrylate monomer (PEG(400)DA) and primary diamine (TTD) were weighed and mixed in dichloromethane (DCM) (equivalent to total monomer mass). This solution was transferred to an aluminum dish and monomers were allowed to react for 24 hours at 50°C in an oven. PβAE gels films were then washed in anhydrous acetonitrile 4 times with a cycle of 1-hour each to remove unreacted monomers followed by freeze-drying to eliminate any residual solvent. The initial ratio of total acrylate to reactive hydrogen amine groups (RTAAP) was varied from 0.6 to 1.65 to synthesize different grades of PβAE gels films (Table 1) Note: 1 mol of diacrylate monomer corresponds to 2 mol of reactive acrylate groups; 1 mol of primary diamine monomer corresponds to total 4 mol of primary/secondary reactive amine groups.
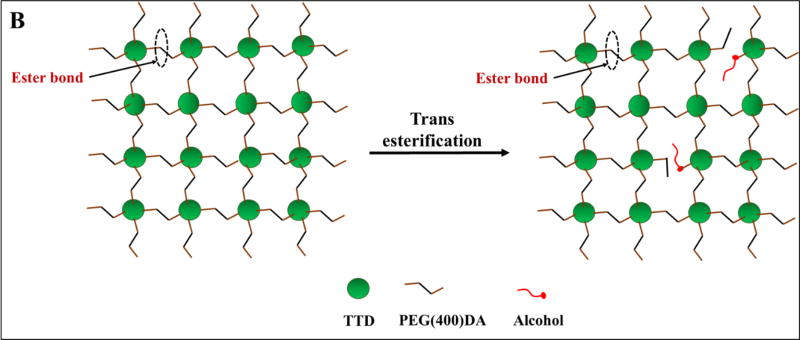
Figure 1. Synthesis of PEGDA-TTD PβAE cross-linked gels.
A. Reaction scheme of PβAE gel formation via reaction of PEG(400)DA and TTD in DCM solvent.
B. Schematic of transesterification of PβAE gels in presence of an alcohol. Ester bond of the PβAE matrix undergoes substitution with the reacting alcohol forming another ester bond.
Table 1.
Different grades of PβAE gels films were synthesized by varying ratio of total acrylate to amine protons (RTAAP)
| Gel Identification Code |
Diacrylate | Diamine | RTAAP |
|---|---|---|---|
| R- 0.6 | PEG(400)DA | TTD | 0.6 |
| R-1.2 | PEG(400)DA | TTD | 1.2 |
| R-1.65 | PEG(400)DA | TTD | 1.65 |
4.2 PβAE degradation in alcohols
Degradation of PβAE gels was carried out in various alcohols at 37°C. 1 cm diameter discs were cut from the bulk gel films, weighed and then incubated in different alcohols of interest. At given time points, discs were removed from the solvent and dried under vacuum to remove residual solvent. The fraction of mass remaining was calculated from the ratio of the recorded final dry mass (Wd) and initial (W0) mass values. The swelling ratio of the selected gel-alcohol incubation system was also calculated by recording the mass of alcohol swollen gels during degradation. Swelling ratio was calculated as the ratio of swollen gel mass (Ws) with initial mass (W0).
A separate set of R-0.6 gels were degraded completely in ethanol as well as in water. Degradation products were freeze dried under vacuum and were subjected to FTIR analysis.
4.3 Dynamic mechanical analysis (DMA)
TA instruments Q800 dynamic mechanical analyzer was used to perform dynamic mechanical analysis in tensile mode. Storage modulus (E’) and tan δ was recorded as a function of temperature at the heating rate of 1 °C/min. The temperature range used was from −70°C to 20°C at 1 Hz. Samples used were in the form of film strips with dimensions of 15 mm*3 mm*0.3 mm (length*width*thickness). All measurements were carried out under inert N2 atmosphere.
4.4 Conjugation of PβAE gels with Riboflavin
R-0.6 and R-1.2 PβAE gel discs were cut in 1-cm discs, weighed and incubated in 1mg/ml riboflavin-DMSO solution at 37°C and allowed to react for various durations of time up to 48 hours. After 6, 12, 24 and 48 hours, gels were taken out, washed twice in DMSO to remove unreacted riboflavin followed by a single wash in anhydrous acetonitrile in order to leach out residual DMSO. The gel discs were then freeze-dried overnight to remove any residual solvents. The next day, each gel disc sample was placed in separate wells of a 12-well plate and read at 444 and 600 nm using Varian Cary 50 Bio UV-Vis spectrophotometer. Absorbance at 600 nm was subtracted from absorbance value at 444 nm as a baseline correction to obtain the absorbance only due to riboflavin conjugation and not the disc thickness/optical density.
Next, the riboflavin conjugated gel discs were degraded in PBS (35 mg gel/ ml of PBS) for gels to undergo hydrolysis and release pure riboflavin. The degradation products in PBS were analyzed once again under UV-Vis at 444 nm and riboflavin concentrations were calculated using standard calibration curve.
5. Results
5.1 PβAE gel synthesis
PβAE gels synthesized with RTAAP of 0.6 (R-0.6) and 1.2 (R-1.2) resulted in uniform gels while gels synthesized with RTAAP of 1.65 (R-1.65) resulted in partial conjugation and hence a semi-solid product. Since a properly conjugated gel wasn’t obtained with R-1.65, further analysis of transesterification was limited to R-0.6 and R-1.2. Post synthesis, gels were washed in acetone to remove unreacted products and mass loss analysis was performed by weighing the gels before and after washing (freeze-dried). Average mass loss of 4.87 ± 0.3% for R-0.6 and 13.1 ± 0.4% for R-1.2 was calculated.
5.2 PβAE degradation in alcohols (mass loss and swelling ratio)
5.2.1 Effect of RTAAP on degradation and swelling ratio using ethanol as a degradation medium
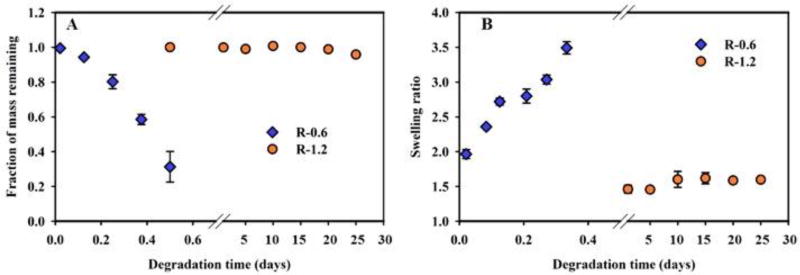
R-0.6 degraded via transesterification in 12 hours with the swelling ratio of 3.5 ± 0.08 at 8 hours. After 8 hours of incubation, the gel started to lose mechanical integrity, suggesting the final stage of complete degradation, as shown in figure 2 A and 2 B. On the other hand, R-1.2 showed very slow kinetics of transesterification derived gel degradation, with no effective mass loss observed in 1st 12 hours. Even after 25 days of ethanol incubation, fraction of mass remaining was calculated to be 0.96 ± 0.01, which is 4% mass loss. R-1.2 did show a swelling ratio of 1.46 ± 0.01 after the 1st day but only increased to 1.65 ± 0.02 after 25 days implying extremely slow degradation kinetics in ethanol.
Figure 2. Effect of RTAAP on transesterification controlled degradation of PβAE gels in 200-proof ethanol at 37° C.
A.) Mass loss of PβAE gels (R-0.6 and R-1.2) presented as a fraction of mass remaining w.r.t. time. B.) Swelling ratio of R-0.6 and R-1.2 in ethanol w.r.t. time. Gels synthesized with RTAAP of 0.6 showed faster degradation and swelling than gels with RTAAP 1.2. N=3, error bars: std. dev.
A similar effect of unsubstituted amines on gel degradation, with water as the degradation medium has been reported earlier. PEGDA-TTD gels synthesized with RTAAP of 0.6 degraded within 40 minutes while gels with RTAAP of 1.2 degraded completely in 130 minutes [19].
5.2.2 Effect of alkyl chain length and degree of alcohol substitution on degradation kinetics
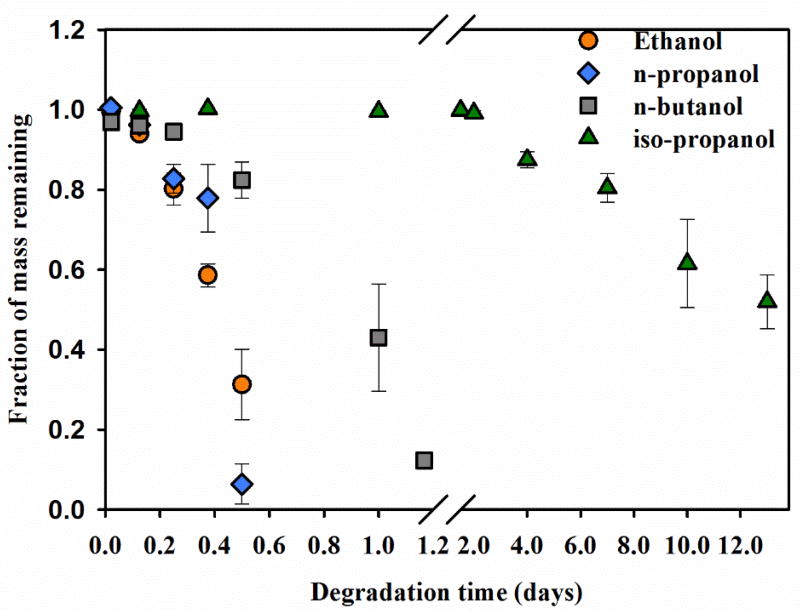
As R-1.2 showed extremely slow degradation kinetics in ethanol, R-0.6 PβAE gels were used to determine the impact of primary alcohols of different chain aliphatic lengths i.e. ethanol, n-propanol and n-butanol. As observed in figure 3, the degradation rate in ethanol and n-propanol was extended over 9–12 hours. However, n-butanol mediated degradation of R-0.6 was slower extending up to 28–30 hours
Figure 3. Degradation of PβAE gels in primary alcohols of different chain lengths and iso-propanol (secondary alcohol).
R-0.6 PβAE gel was incubated in ethanol, n-propanol, n-butanol and iso-propanol at 37° C. N=3, error bars: std. dev.
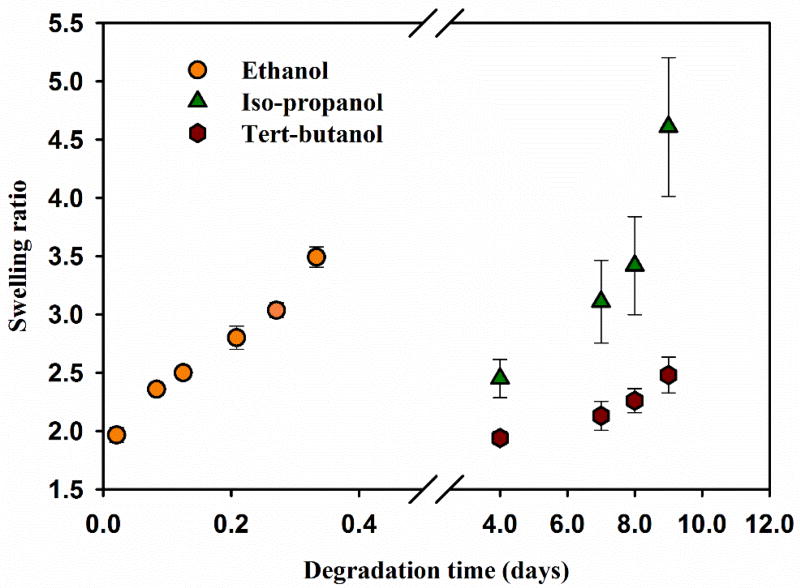
To elucidate the mechanism of gel degradation, the impact of the degree of alcohol substitution (primary, secondary, or tertiary) on degradation rate was conducted in two separate studies. First, R-0.6 degradation was evaluated with alcohols containing the same number of carbons but different degree of substitution i.e. n-propanol (primary, 3-carbon) and iso-propanol (secondary, 3-carbon) (figure 3). It was observed that PβAE gel incubation in primary alcohol (n-propanol) degraded within 12 hours, which is significantly faster than in secondary alcohol (iso-propanol) showing only 50% mass loss in 13 days. In the second study, R-0.6 was incubated in primary, secondary and tertiary alcohol with different chain lengths. Ethanol (primary, 2-carbon), iso-propanol (secondary, 3-carbon) and tert-butanol (tertiary, 4-carbon) were used for transesterification. Swelling ratio for different alcohols was analyzed instead in this study because the mass loss in tert-butanol was too slow to show significant differences over the study time. As shown in figure 4, ethanol-R-0.6 gel incubation resulted in a swelling ratio of 3.5 ± 0.08 within 8 hours after which it disintegrated. Iso-propanol resulted in slower kinetics giving a swelling ratio of 2.45 ± 0.16 after 4 days and 4.61 ± 0.6 after 9 days. Tert-butanol incubation showed even slower kinetics with swelling ratio of 1.94 ± 0.05 after 4 days and 2.48 ± 0.16 after 9 days. Therefore, PβAE degradation kinetics was faster in ethanol than in iso-propanol than in tert-butanol.
Figure 4. Swelling ratio of R-0.6 PβAE gels in primary, secondary and tertiary alcohols i.e. ethanol, iso-propanol and tert-butanol respectively.
Rate of swelling and degradation decreases with increase in chain length and degree of carbon substitution. N=3, error bars: std. dev.
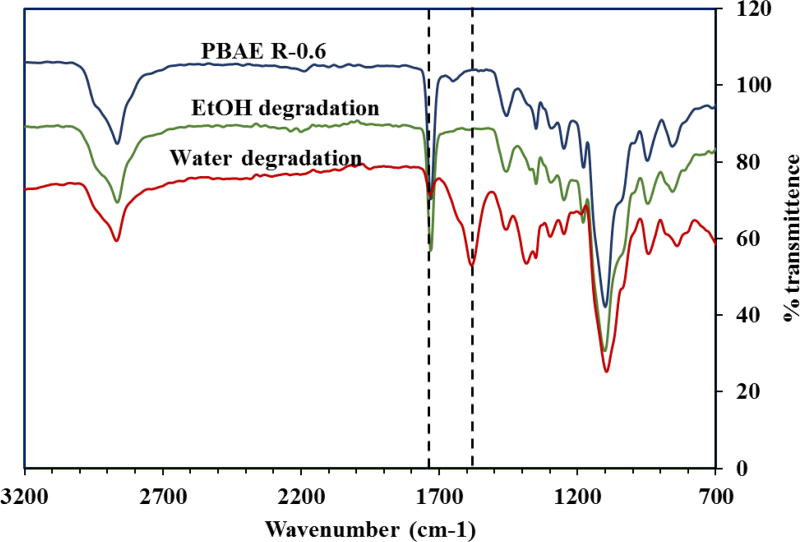
In a separate study, with R-0.6 were hydrolyzed completely in ethanol and water. FTIR analysis of both the systems were compared to identify the nature of degradation products. The degradation products obtained from water showed the presence of strong acid peaks at around 1584 cm−1 clearly indicating the hydrolysis to the corresponding acids. However, the absence of the acid peak (-C=O in carboxylic acid) at 1584 cm−1 and the presence of a ester peak (-C=O in ester) near 1732cm−1 in ethanol degradation products confirmed the formation of the corresponding transesterification products (Figure 5).
Figure 5. FTIR spectra of PβAE gel R-0.6, and the degradation products obtained by the hydrolysis of in water and ethanol.
5.3 Dynamic mechanical analysis (DMA)
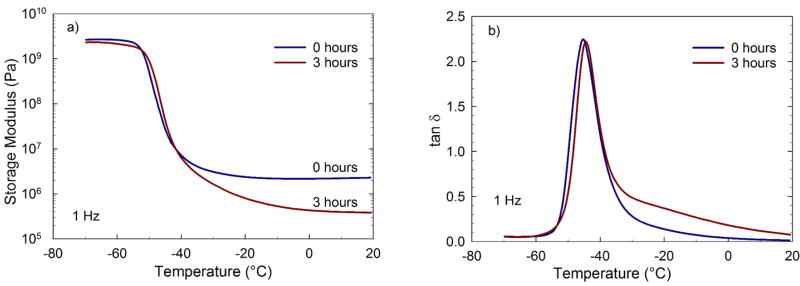
To further assess the impact of ethanol degradation on crosslinking density, DMA was done on RT-0.6 film before degradation (t = 0 hours) and after degradation in ethanol (t = 3 hours). The storage modulus (Figure 6 a) and tan δ (Figure 6 b) as a function of temperature is presented. Samples films were placed in ethanol for 3 hours at 37°C, removed, and fully dried in a freeze drier before evaluating in a dynamic mechanical analyzer. As seen, a step change is seen in the storage modulus as the material undergoes glass-rubber transition. The glass transition region remains unchanged for the samples and is not affected by the degradation. This is evident from tan δ plot too, where both samples have glass transition temperature (Tg) of −45 °C (tan δ peak). Importantly, after 3 hours of incubation in ethanol, the rubbery modulus for the degraded polymer sample is nearly an order of magnitude lower than non-degraded sample.
Figure 6. a) Storage modulus (E’) as a function of temperature and b) tan δ as a function of temperature for RT-0.6 non-degraded gel and gel degraded in ethanol.
A decrease in rubbery modulus is seen due to decreased crosslink density caused by degradation.
5.4 Riboflavin conjugation to PβAE via transesterification
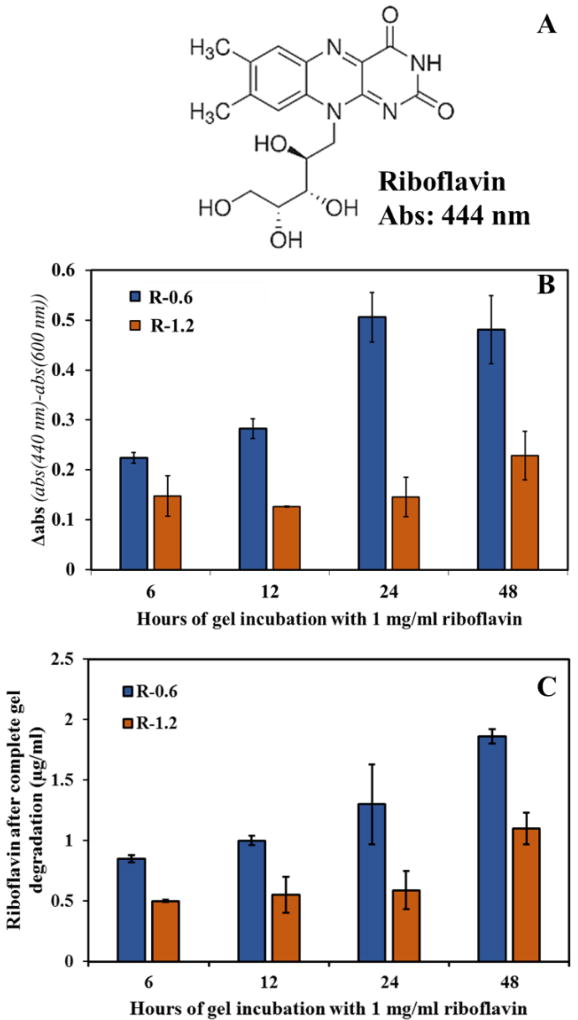
To study whether the transesterification mechanism can be used to link primary alcohol containing compounds to already formed PβAE gels, naturally found riboflavin (vitamin B2) with one primary and three secondary alcohol functional groups was used as a model compound to conjugate into R-0.6 and R-1.2 via transesterification process (figure 7 A). After incubating the gels in 1 mg/ml of riboflavin solution in DMSO for 6, 12, 24 and 48 hours, it was observed that R-0.6 showed higher conjugation than R-1.2 as seen in figure 7 B. Discs were observed under UV-Vis, absorbances were collected at 444 nm i.e. the characteristic peak wavelength of riboflavin and also at 600 nm. Absorbance value at 600 nm is the measure of optical density for each gel due to its thickness. Hence, to get absorbance values due to only riboflavin, absorbance at 600 nm was subtracted as a baseline from 444 nm and presented as Δabs. R-0.6 showed Δabs of 0.22 ± 0.02, 0.28 ± 0.04, 0.5 ± 0.05 and 0.48 ± 0.06 after 6, 12, 24 and 48 hours of incubation respectively. R-1.2 had lower Δabs values of 0.15 ± 0.5, 0.13 ± 0.01, 0.14 ± 0.5 and 0.23 ± 0.05 at the same time points (figure 7 B), which is lower than Δabs for R-0.6.
Figure 7. Conjugation of riboflavin into R-0.6 and R-1.2 PβAE gels by incubation in 1 mg/ml riboflavin solution in DMSO.
A.) Riboflavin structure showing the hydroxyl groups as potential sites for transesterification. B.) abs of the gel discs measured after different time intervals of incubation in riboflavin containing DMSO at 37° C. Δabs = abs (444nm)-abs (600nm). Baseline subtraction at 600 nm was done for each sample separately to void the effect of optical density due to gel thickness. C.) Concentration of riboflavin in PBS calculated after analyzing the degradation products under UV-Vis at 444 nm. The amount of riboflavin conjugation complies with transesterification data obtained earlier in figure 2 showing overall higher transesterification with R-0.6 than R-1.2. N=3, error bars: std. dev.
The riboflavin-conjugated gels were then subjected to hydrolytic degradation in PBS to recover the riboflavin. Degradation products were again read at 444 nm and collected absorbances gave a concentration of riboflavin in the solution using standard curve (figure 7 C). 0.85 ± 0.03, 1 ± 0.04, 1.3 ± 0.33 and 1.86 ± 0.06 µg/ml of riboflavin was retrieved with R-0.6 gels incubated for 6, 12, 24 and 48 hours respectively followed by PBS degradation. For R-1.2, a lower amount of riboflavin was recovered as conjugation was less, which was 0.5 ± 0.01, 0.55 ± 0.15, 0.59 ± 0.16 and 1.1 ± 0.13 respectively under time interval degradation as for R-0.6.
6. Discussion
The degradation studies of PEG(400)DA-TTD based PβAE gel synthesized at different RTAAPs were carried out in ethanol at 37°C. It was initially observed that PβAEs synthesized using single step approach were unstable in ethanol. This was different from previously reported data, where ethanol was used as a stable washing solvent [20]. Originally, it was believed that degradation was due to the water content in the solvent. However, degradation still proceeded even with anhydrous solvents. This observation led to the hypothesis that, β-amino ester bonds in the PβAE gels undergo transesterification with the alcohol to form a new ester.
It was hypothesized that if transesterification was a mechanism, then the unmodified amines contained within the network could serve as a base to catalyze the transesterification process undergoing SN2 reaction. To test this hypothesis, the levels of unmodified amine were varied by changing the total acrylate to total reactive hydrogen amine protons (RTAAP: 0.6 and 1.2). Lower RTAAP (0.6 in this case) PβAE gels with higher unreacted amine content, catalyzed the transesterification reaction via activation of surrounding alcohol into a nucleophilic entity (C2H5OH to C2H5O−) [21]. The activated form, C2H5O− resulted in faster disintegration of PβAE matrix via substitution at the ester linkage (Figure 2). As additional validation, mechanical properties were also found to change with incubation in ethanol. As seen in Figure 6 a, a dramatic decrease in rubbery modulus was observed as a result of degradation in ethanol. This decrease in rubbery modulus is likely due to a decrease in crosslink density of gel caused by breaking of bonds during the degradation process. Also, the glass-rubber transition for degraded sample is slightly broadened (Figure 6 b). This broadening could be due to partial degradation of gel resulting in to mixture of chain fragments or network fragments of different length. The glass transition temperatures of the samples is not affected after 3 hours of degradation. This could be due to significant proportion of gel network backbone, which contributes to the glass transition, still remaining intact.
Additionally, if the degradation of the PβAE gels is a function of transesterification, then it would be expected that the rate of degradation would be dependent upon the reactivity of the alcohol. Hence, the rate of alcohol substitution will be different for alcohols of different chain lengths or degree of carbon substitution. To verify this hypothesis, degradation properties of PβAE gels were analyzed with different alcohols at 37°C (Figures 3 and 4). It was observed that the degradation of R-0.6 PβAE occurred faster in ethanol and n-propanol than in n-butanol amongst the three primary alcohols. The ethanol based transesterification reaction is faster due to the high polar and acidic nature or lower carbon induced inductive effect due to shorter carbon chain length [22]. As the length of the carbon chain increases, nucleophilicity of the alkoxide anion decreases leading to a decrease in the reactivity of alkoxide anion. This causes the slower reaction rate in the order of n-butanol, n-propanol and ethanol and hence the degradation kinetics (Figure 3). A similar trend was seen with the PβAE gel degradation in primary, secondary and tertiary alcohols because of the same concept of higher inductive effect with secondary as compared to primary alcohols. Therefore, iso-propanol being less acidic than n-propanol, shows slower transesterification kinetics than n-propanol (Figure 3). The combined effect of chain length and degree of substitution was seen with degradation carried out in ethanol, iso-propanol and tert-butanol (Figure 4). Here, slowest degradation was observed with tert-butanol amongst all the alcohols used because of the presence of three alkyl groups on the carbon-atom with –OH group and hence least acidic nature.
To further confirm transesterification as the means of PβAE gel degradation, the degradation products of the R-0.6 obtained from water and ethanol were freeze dried and analyzed with the help of an ATR-FTIR (Varian e-spectrophotometer). It has been reported previously that PβAE gels degrade via hydrolysis of ester groups in the crosslinks to lower molecular weight degradation products and chains of poly(β-amino acids) and diols [2, 23]. The presence of a broad acid peak at 1584 cm−1 in the water degradation products of R-0.6 with a subsequent decrease in intensity of the ester-C=O peak at 1732 cm−1 clearly indicates the formation of low molecular weight acids (Figure 5). The transesterification reaction of PβAE with alcohols was also confirmed by the re-formation of new ester peaks at around 1732cm−1 from the ethanol degradation products of R-0.6.
Taking advantage of transesterification of PβAE network by alcohols, compounds of medicinal value containing alcohol groups (e.g. riboflavin, ascorbic acid) can be conjugated to the PβAE network without the use of additional linkers or reagents. Riboflavin is a part of the Vitamin B family (Vitamin B2), naturally found in milk, yeast, leafy vegetables with metabolic functional significance in activating other vitamins and reaction with flavoproteins [24, 25]. It contains one primary and three secondary alcohol groups in the molecular structure (Figure 7 A).
The extent of riboflavin conjugation into PβAE could be analyzed using UV-Vis because of its characteristic wavelength at 444 nm. As shown in Figure 7, PβAE upon incubation in riboflavin solution was able to conjugate the alcoholic molecule into its matrix and the amount of conjugation increased with time showing the progression of transesterification. Higher conjugation was seen with R-0.6 than R-1.2 because of the faster transesterification kinetics complying with previous studies (Figure 2). This was observed at both levels, riboflavin conjugated gel discs (Figure 7 B) and released riboflavin after complete degradation of the gels (Figure 7 C). The riboflavin conjugation extent was further analyzed by calculating the crosslink density of the R-0.6 polymer using the equation (CD = E′/3RTg) [26], where values of E’ (rubbery modulus) and Tg (glass transition temperature) obtained from the DMA analysis. The degree of transesterification for R-0.6 incubated for 48 hour in riboflavin solution was calculated to be 4.9% based on the average crosslink density of 3.51 mole/cm3. Although, this work demonstrates the possibility of covalently conjugating the drug molecules into bulk gel system via transesterification, conjugation efficiency can be further be explored by allowing longer incubation time, concentration of the incubation medium and considering various other drug molecules with −OH groups.
7. Conclusion
It was found that PβAE synthesized by the single step non-free radical polymerization method degrades in alcohol via a transesterification mechanism. Unreacted primary/secondary amines in the PβAE network catalyze this transesterification reaction and the rate of PβAE degradation in alcohol decreases with increase in RTAAP. The degradation rate of PβAE in alcohol decreases with increasing chain length of alcohol or in order of tertiary<secondary<primary alcohols. Taking advantage of alcohol induced transesterification reaction in the PβAE network, molecules of medicinal value with alcohol groups (e.g. riboflavin) can be covalently conjugated to the PβAE network and the amount to be conjugated can be controlled with incubation time until gel disintegration while release of riboflavin via water hydrolysis can be controlled by selecting the PβAE gels of different RTAAPs.
Acknowledgments
The authors would like to thank Dr. Matthew Weisenberger for assistance with dynamic mechanical measurements completed at University of Kentucky Center for Applied Energy and Research.
References
- 1.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angewandte Chemie-International Edition. 2003;42(27):3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DG, et al. A combinatorial library of photocrosslinkable and degradable materials. Advanced Materials. 2006;18(19):2614-+. [Google Scholar]
- 3.Hawkins AM, et al. Composite hydrogel scaffolds with controlled pore opening via biodegradable hydrogel porogen degradation. J Biomed Mater Res A. 2014;102(2):400–12. doi: 10.1002/jbm.a.34697. [DOI] [PubMed] [Google Scholar]
- 4.Keeney M, et al. Development of Poly(β-amino ester)-Based Biodegradable Nanoparticles for Nonviral Delivery of Minicircle DNA. ACS Nano. 2013;7(8):7241–7250. doi: 10.1021/nn402657d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunshine JC, et al. Poly(beta-amino ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PLoS One. 2012;7(5):e37543. doi: 10.1371/journal.pone.0037543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins AM, et al. Synthesis and analysis of degradation, mechanical and toxicity properties of poly(beta-amino ester) degradable hydrogels. Acta Biomater. 2011;7(5):1956–64. doi: 10.1016/j.actbio.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy D, et al. Poly(Ethylene Oxide)-Modified Poly(β-Amino Ester) Nanoparticles as a pH-Sensitive System for Tumor-Targeted Delivery of Hydrophobic Drugs: Part 2. In Vivo Distribution and Tumor Localization Studies. Pharmaceutical Research. 2005;22(12):2107–2114. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynn DM, Amiji MM, Langer R. pH-Responsive Polymer Microspheres: Rapid Release of Encapsulated Material within the Range of Intracellular pH. Angewandte Chemie International Edition. 2001;40(9):1707–1710. [PubMed] [Google Scholar]
- 9.Biswal D, et al. A single-step polymerization method for poly(beta-amino ester) biodegradable hydrogels. Polymer. 2011;52(26):5985–5992. [Google Scholar]
- 10.Chen J, et al. Synthesis and degradation of poly(beta-aminoester) with pendant primary amine. Polymer. 2007;48(3):675–681. [Google Scholar]
- 11.Patil VS, Dziubla TD, Kalika DS. Static and dynamic properties of biodegradable poly(antioxidant β-amino ester) networks based on incorporation of curcumin multiacrylate. Polymer. 2015;75:88–96. [Google Scholar]
- 12.Meenach SA, et al. Controlled synergistic delivery of paclitaxel and heat from poly(β-amino ester)/iron oxide-based hydrogel nanocomposites. International Journal of Pharmaceutics. 2012;427(2):177–184. doi: 10.1016/j.ijpharm.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Lakes AL, et al. Synthesis and Characterization of an Antibacterial Hydrogel Containing Covalently Bound Vancomycin. Biomacromolecules. 2014;15(8):3009–3018. doi: 10.1021/bm5006323. [DOI] [PubMed] [Google Scholar]
- 14.Jain S, Sharma MP. Kinetics of acid base catalyzed transesterification of Jatropha curcas oil. Bioresource Technology. 2010;101(20):7701–7706. doi: 10.1016/j.biortech.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Singh AK, Fernando SD, Hernandez R. Base-Catalyzed Fast Transesterification of Soybean Oil Using Ultrasonication. Energy & Fuels. 2007;21(2):1161–1164. [Google Scholar]
- 16.Rautio J, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 17.Ettmayer P, et al. Lessons Learned from Marketed and Investigational Prodrugs. Journal of Medicinal Chemistry. 2004;47(10):2393–2404. doi: 10.1021/jm0303812. [DOI] [PubMed] [Google Scholar]
- 18.Wattamwar PP, et al. Synthesis and characterization of poly(antioxidant beta-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomaterialia. 2012;8(7):2529–2537. doi: 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Biswal D, et al. A single-step polymerization method for poly(β-amino ester) biodegradable hydrogels. Polymer. 2011;52(26):5985–5992. [Google Scholar]
- 20.Fisher PD, et al. Improved small molecule drug release from in situ forming poly(lactic-co-glycolic acid) scaffolds incorporating poly(beta-amino ester) and hydroxyapatite microparticles. J Biomater Sci Polym Ed. 2014;25(11):1174–93. doi: 10.1080/09205063.2014.923368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otera J. Transesterification. Chemical Reviews. 1993;93(4):1449–1470. [Google Scholar]
- 22.Kim M, et al. Competitive transesterification of soybean oil with mixed methanol/ethanol over heterogeneous catalysts. Bioresource Technology. 2010;101(12):4409–4414. doi: 10.1016/j.biortech.2010.01.099. [DOI] [PubMed] [Google Scholar]
- 23.Brey DM, Burdick JA. POLY 552-Biodegradable poly(beta-amino ester)s as substrates for mineralized tissue formation. Abstracts of Papers of the American Chemical Society. 2008;236 [Google Scholar]
- 24.Powers HJ. Riboflavin (vitamin B-2) and health. The American Journal of Clinical Nutrition. 2003;77(6):1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 25.Aratani Y, et al. Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus. Med Mycol. 2002;40(6):557–63. doi: 10.1080/mmy.40.6.557.563. [DOI] [PubMed] [Google Scholar]
- 26.Song L, et al. Synthesis and evaluation of novel dental monomer with branched carboxyl acid group. Journal of biomedical materials research. Part B, Applied biomaterials. 2014;102(7):1473–1484. doi: 10.1002/jbm.b.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]