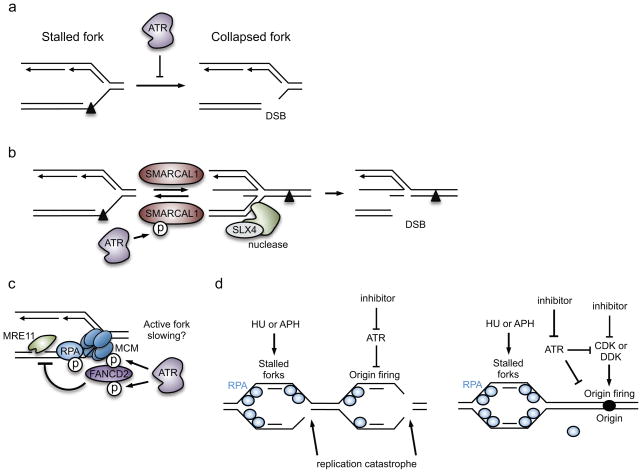
Figure 5. Proposed mechanisms by which ATR maintains replication-fork stability.
(a) ATR prevents fork collapse, which is illustrated here as double-strand DNA break (DSB) formation at the fork. In this example, replication of the leading strand was blocked by a DNA lesion. (b) ATR phosphorylates SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a like 1 (SMARCAL1) at stressed replication forks, thereby suppressing its activity and limiting fork reversal. As structure-specific endonuclease subunit (SLX4)-dependent nucleases can cleave reversed forks, ATR-dependent inhibition of SMARCAL1 is a mechanism by which ATR stabilizes the fork. (c) ATR phosphorylates several proteins at the replication fork to modify replisome function. ATR mediates the recruitment of Fanconi anemia complementation group D2 (FANCD2) to the fork, which may occur in response to ATR-dependent phosphorylation of the MCM2-7 helicase and FANCD2. FANCD2 minimizes the accumulation of ssDNA caused by meiotic recombination 11 homolog A (MRE11)-dependent resection of the nascent DNA. FANCD2 also slows DNA polymerases at stressed forks, and fork slowing may prevent collapse. (d) ATR prevents exhaustion of replication protein A (RPA) availability and subsequently replication catastrophe. This indirect function of ATR is mediated partly through its role in suppressing origin firing in response to hydroxyurea (HU)- or aphidicolin (APH)-induced replication stress.