Abstract
Two new tetracationic hetero-bimetallacycles, 4 and 5, have been constructed from an N,N′-bis(4-(pyridin-4-ylethynyl)phenyl)pyridine-2,6-dicarboxamide ligand, 1, and cis-blocked complexes [M(dppf)](OTf)2 (dppf = 1,1′-bis(diphenylphosphino)ferrocene; M = Pd (2), Pt (3)) in CH3NO2/CH2Cl2 (1:1) solvent. Both complexes were isolated with adequate yields as triflate salts and were then characterized using 1H, 13C, and 31P NMR spectroscopy, elemental analysis, UV-Vis spectroscopy, and high-resolution electrospray mass spectrometry (HR-ESMS). The molecular structure of 4 was determined by molecular mechanics force field calculations. The cytotoxic effect of both new complexes were analyzed against T98G (brain tumor), KB (head and neck cancer), SNU-80 (thyroid cancer), and HEK-293 non-malignant cell lines. The cytotoxicity of complexes 4 and 5 were found to be considerably more effective against cancer cells than reference drug cisplatin. Annexin-V/PI staining, caspase-3/-7 activity, reduction in mitochondrial membrane potential justify a significant level of apoptosis in complex treated cells.
Keywords: Supramolecular, self-assembly, hetero-metallacycle, pyridine-amide ligand, anticancer activity
Introduction
Coordination-driven self-assembly has proven to be an efficient approach towards the synthesis of large supramolecular metallacyclic structures with well-defined shapes and sizes.[1] Wide arrays of molecular architectures with various aesthetically pleasing shapes, sizes, and symmetries have been reported using a coordination-driven directional bonding approach that combines rigid, ligand-capped transition metal acceptors and mono- or multidentate donors bearing O- and N-coordinating sites.[2] Square, planar Pd(II) and Pt(II) organometallic acceptors with pyridyl or nitrogen donor linkers are most extensively used for the design of such metallacyclic structures because of their rigid square-planar coordination environments.[3] Organic amides bearing nitrogen donor symmetrical polypyridyl ligands have been widely used in coordination-driven self-assembly.[4] These self-assembled supramolecular architectures have been exploited for a variety of applications in host-guest, catalysis and sensing, etc.[5] More recently, the biological properties of these metallacyclic structures have also begun to emerge. Metallacycles[6] and helicates[7] have been found to interact with DNA, while other architectures have been shown to be cytotoxic to cancer cells and bacteria.[8]
On the other hand, the metallopharmaceutical has emerged as an important field of medicinal chemistry due to the therapeutic applications of metal-based drugs.[9,10] Usually, arrangements of organic ligands in a two- or three-dimensional space, from the wide range of coordination numbers and accessible redox states of tunable metal centers, offer a wide spectrum of reactivity that can be utilized for medicinal purposes.[11] Since the discovery of cisplatin, there has been a vast expansion of interest in the use of inorganic compounds for the treatment of tumors. Despite its successful application as a therapeutic agent for a wide range of cancers, cisplatin use also has a number of negative side effects, such as high toxicity and undesirable nephrotoxicity (kidney damage), neurotoxicity (damage to the nervous system), and myelotoxicity (bone marrow suppression).[12] The efficacy of cisplatin can also be diminished by intracellular degradation and resistance. These drawbacks have led to the development of other platinum drugs, such as carboplatin, oxaliplatin, and satraplatin, which have lower toxicity and are currently used for the treatment of various cancers.[13] While these derivatives exhibit improved therapeutic properties compared to cisplatin, issues associated with these platinum drugs remain. Therefore, considerable effort is being put into the development of drug delivery vectors that can alleviate these issues related to toxicity, degradation, and resistance. Therrien and co-workers have reported a half-sandwich, ruthenium-based coordination cage that encapsulates the [M(acac)2] (M = Pt2+, Pd2+) complexes and synergistically enhances their antitumor activity.[14]
Recently, we have reported various molecular metallacycles constructed by the coordination-driven self-assembly approach that were evaluated for in vitro anti-cancer activity.[15] The results suggested that larger metallacycles express higher activity levels than small metallacycles, which supports the hypothesis that large macromolecules are more readily retained inside cancer cells.
Inspired by this finding and with the intention of building on our expertise in metallosupramolecular structures, we have designed and synthesized two new large hetero-bimetallacycles (HBMCs) by an amide-based dipyridyl ligand with two different metal (Pd and Pt) acceptors. To explore the potential biological effects of the interaction of these systems with biomolecules, we evaluated the anti-cancer efficacies of HBMCs, along with the starting acceptors and donor ligands, against T98G (brain tumor), KB (head and neck cancer), SNU-80 (thyroid cancer), and HEK-293 non-malignant cell lines.
Results and Discussion
Synthesis and Characterization of Hetero-Bimetallic Metallacycles (HBMCs)
As shown in Scheme 1, the HBMCs 4 and 5 were prepared by a reaction between bis(4-(pyridin-4-ylethynyl)phenyl)pyridine-2,6-dicarboxamide)) donor ligand 1 and heterometallic cis-M[(dppf)(OTf)2] [M = Pd (2), Pt (3); dppf = 1,1′-bis (diphenylphosphino) ferrocene; OTf = trifluoromethanesulfonate] acceptors in a [2+2] manner.
Scheme 1.
Synthesis of Hetero-Bimetallic Metallacycles (HBMCs)
NMR spectroscopy was initially used to characterize the metallacycles. The 1H NMR spectra of 4 and 5 clearly show the formation of symmetrical species by typical resonance shifts due to metal complexation. Compared to the proton signals in donor 1, we found that upon coordination, all of the signals in both metallacycles 4 and 5 shifted significantly (Figures S1 and S2).
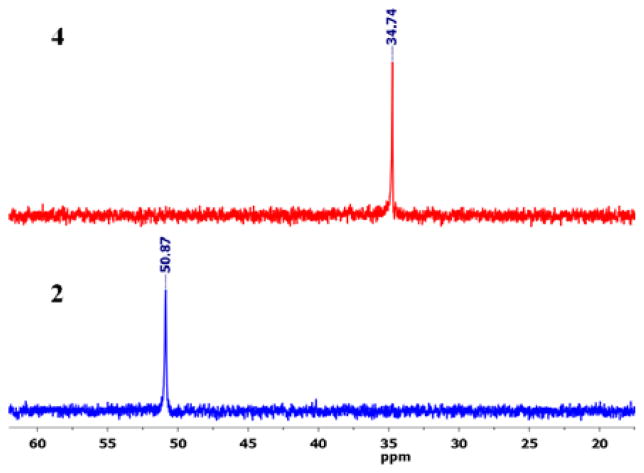
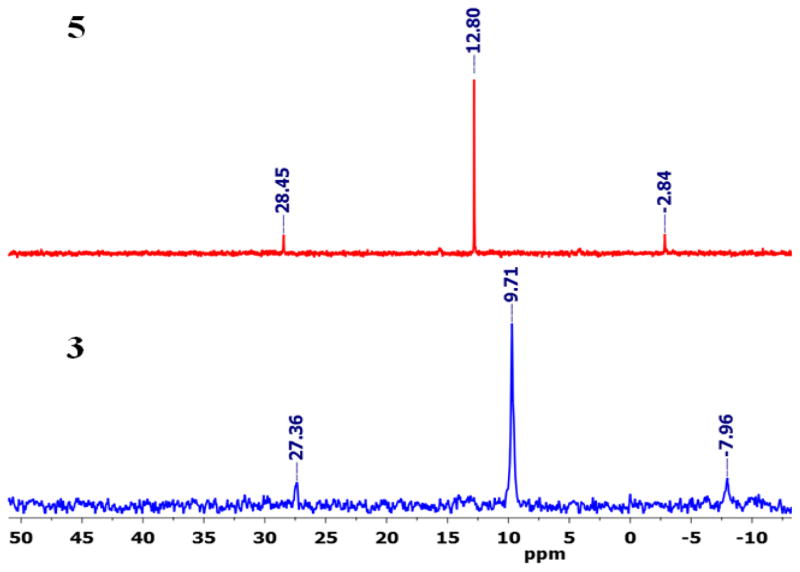
The 31P NMR spectrum of 4 showed one sharp peak at 34.74 ppm that was shifted upfield ~16.5 ppm compared to the signal of the starting acceptor due to metal coordination with the pyridines (Figure 1). The appearance of a single 31P signal was indicative of the presence of symmetric phosphorus nuclei. However, the 31P NMR spectrum of 5 displayed a sharp singlet at 12.80 ppm along with 195Pt satellites, indicating the formation of a single product (Figure 2).
Figure 1.
Comparative 31P NMR spectra of HBMC 4 and Pd acceptor 2 in nitromethane-d3.
Figure 2.
Comparative 31P NMR spectra of HBMC 5 and Pd acceptor 3 in nitromethane-d3.
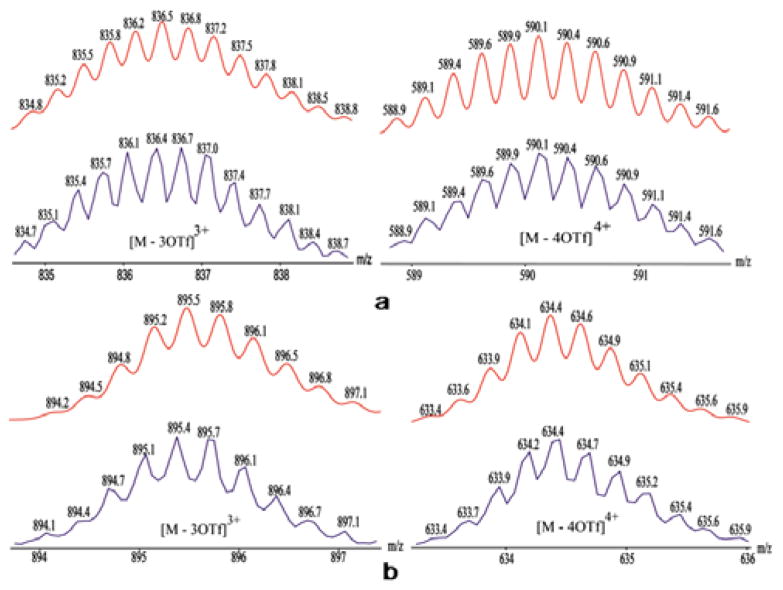
Electrospray ionization mass spectrometry (ESI-MS) provided further evidence for the formation of metallacycles. The charged states for assembly 4 at m/z = 836.4 ([M-3OTf]3+) and 590.1 ([M-4OTf]4+) (Figure 3a), and assembly 5 at m/z = 895.4 ([M-3OTf]3+) and 634.4 ([M-4OTf]4+) were clearly observed and isotopically resolved (Figure 3b). These peaks matched well with the theoretical distributions.
Figure 3.
Calculated (upper) and experimental (lower) ESI-MS spectra of HBMC 4 (a) and 5 (b).
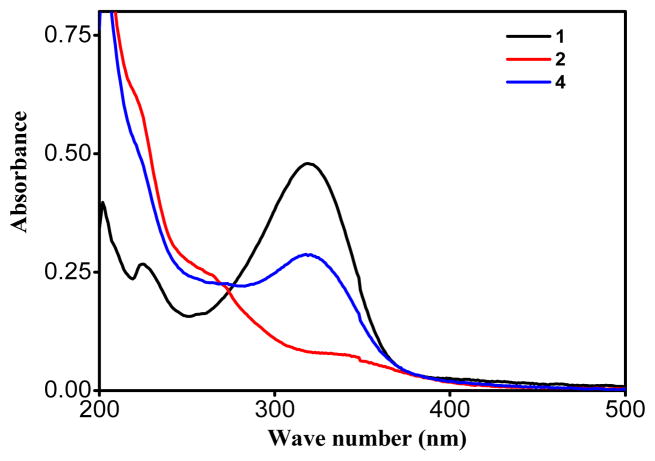
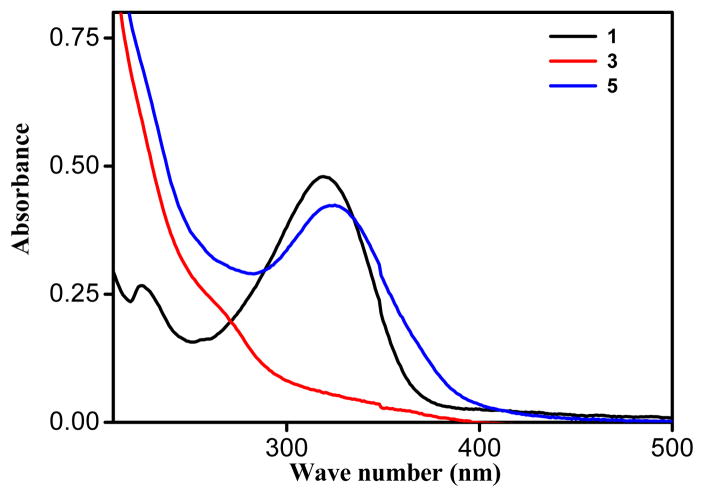
Electronic absorption spectra of the metallacycles 4 and 5 as well their acceptors were acquired in methanol solutions at 1×10−5 M (Figures 4 and 5). Strong absorption was observed at 320 nm in metallacycle 4, with a considerable blue-shift of the d-d bands compared to the initial Pd-acceptor (Figure 4). The difference in λmax position for metallacycle 4 and Pd-acceptor 2 was clearly due to the strong-field coordination of the nitrogen lone pair electrons on the pyridine ring in the metal’s center. Similarly, in metallacycle 5, a strong absorption was observed at 325 nm, with a considerable blue-shift compared to that of the starting Pt acceptor (Figure 5).
Figure 4.
Comparative UV-Vis spectra of ligand 1, Pd acceptor 2, and HBMC 4.
Figure 5.
Comparative UV-Vis spectra of ligand 1, Pt acceptor 3, and HBMC 5.
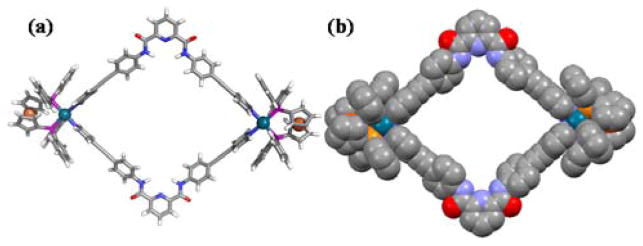
Finally, the structure of [2 + 2] self-assembled HBMC 4 was determined using molecular mechanics force field calculations. A geometry optimization was carried out using UFF force fields, as described by Rappe and coworkers,[16] using the Gaussian 09 computational software suite.[17] Individual fragments were initially optimized with chlorides, completing the coordination sphere of the Pd centers. These fragments were then combined into full rhomboids and subsequently optimized, culminating in the geometry shown in Figure 1. The hydrogen atoms of the cyclopentadienyl rings were added to their calculated optimized positions. The Pd centers are separated by a distance of 23.8 Å, and the pyridyl-nitrogen atoms of the central rings of the bridging ligands are separated by 19.5 Å. Though the structure is largely planar, the central pyridyl rings are canted up from the plane defined by the coordination sphere of the Pd centers, with torsion angles of 24.2° and 10.2°, contributing to the rhomboid’s slightly bowl-like character.
Inhibitory effects of ligands and HMBCs on cancerous and normal cells
Metal-based drugs are widely used in clinical applications.[18, 19] To explore the potential biological effects of the interaction of these systems with biomolecules, we have evaluated ligand 1, acceptors 2 and 3, and new HBMCs 4 and 5 for their cytotoxicity against T98G (brain tumor), KB (head and neck cancer), SNU-80 (thyroid cancer), and HEK-293 non-malignant cell lines. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTT), a marker of proliferation, was reduced by viable cells to a colored formazan product via metabolic dehydrogenase enzymatic activity. The MTT cell proliferation assay has been widely accepted as a reliable way to measure the cell proliferation rate and, conversely, when metabolic events lead to apoptosis or necrosis. For these reasons, MTT assays are used for chemo and radiosensitivity testing. The data obtained by the MTT assay show that all complexes had inhibitory effects on the growth of cancer cells in a dose-dependent manner. Complex 4 and 5 effectively inhibited the growth of T98G, KB, and SNU-80 cells, with IC50 values ranging from 4.5 – 18.3 μM (Table 1). Cisplatin, a chemo- therapeutic drug, was used as a control. Both complexes 4 and 5 were found to inhibit 50.0% of T98G brain cancer cell growth in the range of 4.5 to 5.2 μM after 24 hour (h) of treatment. The data obtained suggest that the complexes are less cytotoxic to non-malignant HEK-293 cells at effective concentrations (4.5–18.3 μM) against cancer cells (Table 1). IC50 values of the complexes for HEK-293 cells ranged from 35.0 – 36.2 μM. These results indicate that both 4 and 5 inhibit T98G brain tumor cell growth, which may be due to either growth arrest or cell death. Overall, these results clearly demonstrate that complexes 4 and 5 effectively sensitize T98G cells in a dose-dependent manner, which may be due to an increase in mitotic (linked to cytogenetic damage) or interphase (apoptosis) death. Furthermore, we performed a growth kinetics assay, a clonogenic survival assay, an reactive oxygen species (ROS) generation assay, mitochondrial membrane potential (MMP) detection, total intracellular ATP detection, caspase 3/7 activity and annexin V/PI staining assay for apoptosis detection in treated T98G cancer cells to confirm growth inhibition, clonogenic inhibition, oxidative stress, and apoptosis induced by newly synthesized complexes, respectively.
Table 1.
IC50 values (μM) of synthesized metal complexes on T98G brain cancer, KB head and neck cancer, SNU80 thyroid cancer, and HEK293 non-cancerous cells.
| Complex | T98G | KB | SNU80 | HEK293 |
|---|---|---|---|---|
| 1 | 144.0(±17.5) | 124.7(±9.6) | 107.3(±5.1) | 156.6(±9.9) |
| 2 | 143.7(±13.4) | 86.6(±6.9) | 80.1(±7.4) | 90.0(±8.2) |
| 3 | 32.5(±5.2) | 76.6(±6.3) | 70.1(±5.4) | 65.3(±5.3) |
| 4 | 5.2(±2.0) | 15.5(±2.3) | 18.3(±3.0) | 36.2(±5.1) |
| 5 | 4.5(±2.1) | 13.0(±1.2) | 12.0(±2.8) | 35.0(±3.8) |
| cisplatin | 69.6(±7.9) | 72.6(±6.2) | 49.3(±5.4) | 17.3(±3.6) |
Growth Kinetics
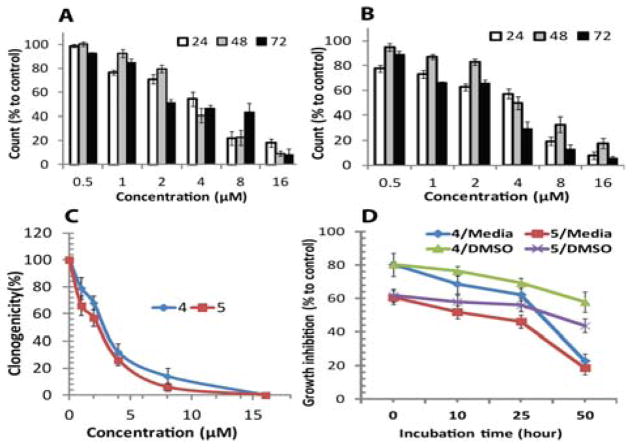
Figure 7(A) and (B) shows the growth kinetics pattern of the T98G cells. Data obtained by growth kinetics assay revealed that complexes 4 and 5 have a significant inhibitory effect on T98G cells in incubation time dependent fashion. Cell suspensions were made in 4 mm layers of media in 6-well plates and the cultured cells were treated with 0.5 to 16 μM concentrations of both complexes and It is important to note that the cells exposed to complex 4 demonstrated a slightly weaker growth inhibitory effect than those exposed to complex 5. The maximum effect was seen after treatment with complex 5, due to inhibition of cell growth from 32.4%–92.4% after 24 h of treatment (viability was 7.6%–77.6%). In the case of T98G cells exposed to complex 4, we found that 2%–81.8% of cells died, and their viability was therefore recorded as 18.2%–98.0% after 24 h of treatment at various doses. Overall, 4 and 5 inhibited the growth of T98G cells in a dose and incubation-dependent manner. These experiments indicate that both complexes inhibit T98G cancer cell growth and that inhibition may be due to either growth arrest or cell death.
Figure 7.
Inhibitory effect of complex 4 and 5 on T98G solid brain tumor cells. Percentage of viable T98G cells counts after treatment by (A) complexes 4, and (B) complex 5 at 24, 48 and 72 h. Data obtained by growth kinetics assay (A and B) showed that complexes 4 and 5 have an inhibitory effect on T98G cells that is dependent upon incubation time. The cultured cells were treated with 0.5 to 16 μM concentrations of the complexes. (C) Clonogenic capacity of T98G cells treated with complex 4, and 5. (D) Loss of growth-inhibiting activity of the complexes pre-incubated in culture medium. Complexes 4 and 5, at a concentration of 6 μM, were pre-incubated in DMSO and cell culture medium (DMEM) supplemented with 10% FBS at 37°C for the indicated times before being added to cultures of T98G cells.
Inhibition of colony formation capacity of cells
Figure 7(C) shows the effect of the complexes on the colony-forming capacity of T98G cells. Only a fraction of seeded cells retained the capacity to produce colonies after cytotoxic drug treatment. Clonogenic in vitro method for measuring clonogenicity is recognized as a valid surrogate assay for tumor growth in vivo. Increased complex concentration enhanced cell death and inhibited the colony formation capability of the T98G cell population. The clonogenic assay demonstrated the effect of complexes 4, and 5 on the colony-forming capacity and survival of exponentially growing T98G cells. It was observed that the surviving fraction of T98G cells was drastically decreased after treatment with synthesized complexes. It is important to note that treatment with complexes 4, and 5 enhanced cell death and also inhibit the colony formation capability of the T98G cell population in a concentration-dependent manner.
The surviving fraction of T98G cells declined as treatment concentrations increased (1–16 μM), which is evident from the reduced number of colonies that formed. Colony survival significantly declined to levels of 79.0% and 66.0% even at a low dose (1 μM) of complexes 4 and 5 respectively. However, the most drastic decline in colony survival was observed after exposure to higher concentrations (> 4 μM) of complexes 4 and 5, and complexes 5 specifically, showed a stronger inhibitory effect against colony formation by T98G cells, at all doses. Complex 5 showed maximum inhibition at higher doses (> 4 μM) and colony survival was observed to be 26.0%, 6.0%, and 0% after exposure to doses of 4, 8, and 16 μM, respectively. These outcomes reflect a significant inhibitory effect on the colony formation capabilities of brain cancer cells at all doses of both complexes tested.
Stability of HMBCs
To determine the stability of 4 and 5, 6 μM solutions of 4 and 5 were pre-incubated in cell culture medium (DMEM) and DMSO for 0, 10, 25, and 50 h at 37°C. Cell growth was examined as described in Methods (MTT cell viability assay) for T98G tumor cells. As shown in Figure 7(D), a 50.0% reduction in the growth-inhibiting activity of complexes 4 and 5 was observed after ~50 h of pre-incubation in cell culture medium. In contrast, in the presence of DMSO, growth-inhibiting activities were stable until ~25 h of pre-incubation. It would be reasonable to expect that the loss of growth-inhibiting activity would parallel the loss of the complexes from the medium, suggesting that complexes 4 and 5 may be stable for <25 h of pre-incubation in the cell culture medium. These results suggest that complexes 4 and 5 are stable in DMSO and cell culture medium, at least during the phase when the complexes have a strong effect on cell viability. For the further stability studies, the stability of complexes has been evaluated under various biological conditions using fluorescence spectroscopy (Figure S6 and S7). Both complexes are stable in physiological buffer solution at pH 7.4. In the presence of oxidative (H2O2) or reductive (dithiothreitol) derivatives, no significant degradation of the complex systems was observed under the experimental condition, thus attesting to the stability of the complexes under physiological conditions.
Reduction in intracellular ATP level
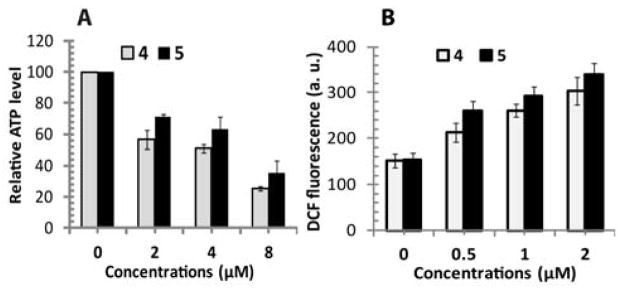
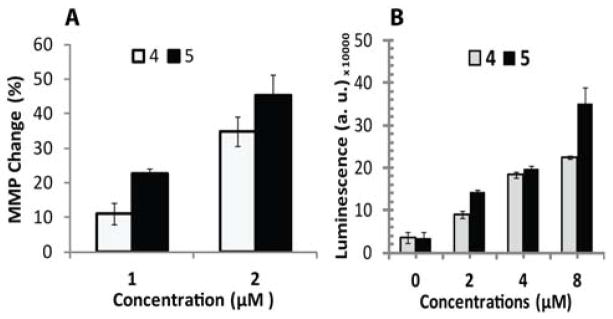
In order to check the effects of complex 4 and 5 on mitochondrial metabolic status, we measured intracellular ATP generation in T98G brain tumor cells that have important roles in cell death. Intracellular ATP was quantified by Promega Ultra-Glo™ recombinant luciferase in serum containing cell culture media. Figure 8(A) shows complex 4 and 5 induced significant changes in ATP production with almost similar tendency. Relative ATP values reduced more with increased concentration of complexes at 24 h. The effect of 0.05 μM concentration of complexes was almost negligible (data not shown); however, higher concentration (2, 4 and 8 μM) induced significant reduction in mitochondrial ATP production in T98G tumor cells.
Figure 8.
Intracellular ATP and reactive oxygen species (ROS) detection. (A) The relative intracellular ATP value of T98G cells in DMEM media. T98G cells exposed to 4 and 5 showed much decreased ATP level and metabolic activity at 24 h following treatment. (B) ROS generation in T98G cells treated with complexes 4 and 5. Data are displayed as the mean fluorescence intensity of DCF using a H2DCFDA probe.
HMBCs treatment enhances ROS level in cells
Intracellular ROS measurement in T98G cells after treatment with synthesized complexes is shown in Figure 8(B). The decreased MTT formazan due to growth inhibition and increased clonogenic inhibition might lead to increased oxidative stress and, therefore, compromised survival of T98G cells. To determine if treatment with synthesized complexes enhanced the steady state of oxidative stress in malignant cells, cells were labeled with the oxidation-sensitive probe (H2DCFDA), which, upon oxidation by ROS, gives off a fluorescent product (DCF) that was measured using a microplate reader with excitation at 485 nm and emission at 528 nm. We found that complexes 4 and 5 enhanced ROS production after 24 h inside T98G cancer cells at 0.5–2 μM concentrations. Treatment with complex 4 increased the DCF fluorescence by 39.2% (MFI-213), 70.5% (MFI-261), and 98.6% (MFI-304), respectively, at concentrations of 0.1, 0.5, 1, and 2 μM in T98G cells, compared to an untreated control (MFI-153). Complex 5 treatments also increased DCF fluorescence by 68.8% (MFI-260), 90.2% (MFI-293), and 120.1% (MFI-339), respectively, at concentrations of 0.5, 1, and 2 μM in T98G cells, compared to an untreated control. However, cells treated with a concentration of 0.1 μM of the complexes showed no significant changes in ROS production (data not shown, as DCF fluorescence this concentration is almost similar to the control). Interestingly, liquid holding conditions had no influence on oxidative stress in the cell lines studied, as the DCF fluorescence of untreated cells was similar in PBS and growth media (MFI in PBS, DCF = 144; MFI in growth media, DCF = 153).
HMBCs induces apoptosis in cells
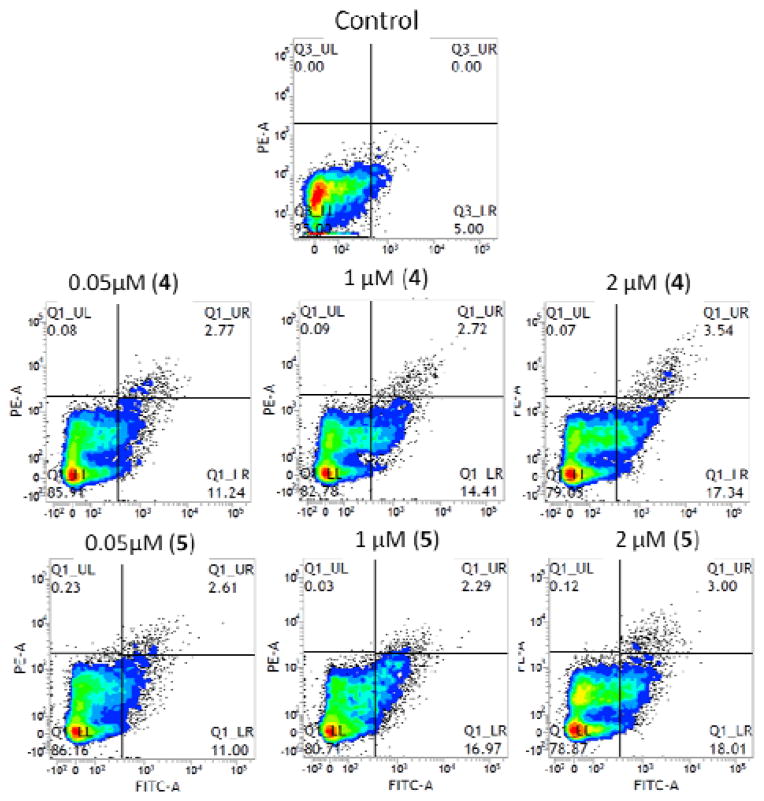
Apoptosis is the best-described type of programmed cell death and is characterized by distinct morphologic features, such as cell membrane blebbing, reduction in cellular volume, activation of caspases, chromatin condensation, and nuclear fragmentation.[20] Since cell survival was dramatically reduced after the treatment, we next evaluated whether the complexes induced apoptosis with flow cytometery analysis (BD FACS Verse). Cell populations were measured by staining with fluorescein isothiocyanate (FITC)-labeled annexin V (green fluorescence) and exclusion of the non-vital dye, propidium iodide (PI). In this manner, we were able to discriminate between intact, early apoptotic, late apoptotic, and necrotic cells.[21] In Figure 9, the lower left quadrants (Q1-LL) of the cytograms show viable cells, excluded PI and were negative for FITC-annexin V binding (FITC−/PI−). The upper right quadrants (Q1-UR) represent late apoptotic, non-viable, necrotic cells, positive for FITC-annexin V binding and PI uptake (FITC+/PI+). The lower right quadrants (Q1-LR) represent apoptotic cells with FITC-annexin V binding and cytoplasmic membrane integrity (FITC+/PI−). All treatments induced significant apoptosis in a dose-dependent fashion in the cells. As shown in Figures 9 and Figure S3 (supporting information), complex 4 and 5 treatment increased annexin V-FITC binding by 2–4 fold. The negative control showed a level of 5.0% annexin V-FITC binding, while 2 μM concentrations of complexes 4 and 5 showed levels of 20.8% and 21.0%, respectively.
Figure 9.
Detection of apoptosis in human T98G brain tumor cells. Cells treated with complexes 4, and 5 at various concentrations (0.05–2 μM) were suspended in binding buffer. Annexin V-FITC and propidium iodide solution were added and cell solutions were incubated for 15 min at room temperature in the dark. Flow cytometric analysis was performed within 1 h. Each number represents the percentage of cells in each quadrant.
Loss of mitochondrial membrane potential and caspase activation are involved in HMBCs induced apoptosis
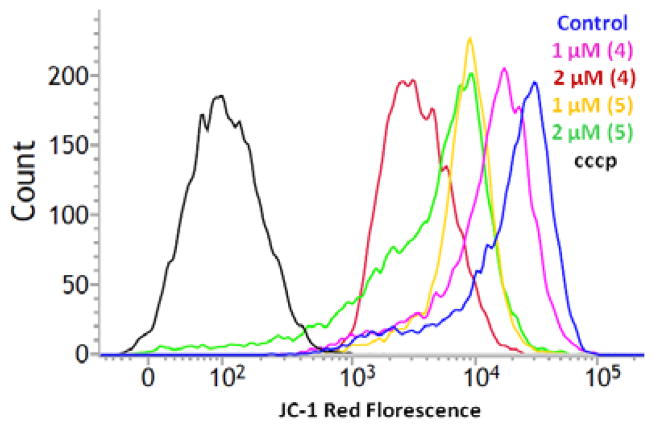
Therapeutic agents and enhanced ROS level often induces mitochondrial membrane permeability change, an early event towards cell apoptosis.[22] Complex 4 and 5 treatment generates reactive species that can disrupt membrane polarization. Under similar treatment conditions, following 24 h of incubation, T98G cells were incubated with JC-1 dye for 30 min; and then, the cells were analyzed by FACS analysis. Figure 10 and 11(A) demonstrates the mitochondrial membrane potential (MMP) change of cancer cells. Membrane potential of 4 and 5 treated T98G cells shows significant change as compare to untreated control. Treatment with 1 and 2 μM concentration of complex 4 induces MMP change by 11.0% and 34.7% respectively in cells. Maximum effect shown by complex 5 treatment, it change MMP by 22.6 % and 45.2% at 1 and 2 μM concentration respectively. Figure 10 show a band shift phenomenon in cells observed after 24 h incubation. Treatment with complex 4 and 5 indicated mitochodrial membrane depolarization within the cells in dose-dependent manner, as an indication of apoptosis. Caspase 3 and 7 play an important role in the real cleavage of cellular constituents during apoptosis. Figure 11(B) shows the caspase activity in both control and treated cells at 24 h post treatment. In untreated cells detected caspase activity level related to the fraction of apoptotic cells existing in the growing population due to natural aging. In treated cells, caspase 3/7 activity increased over basal levels. Complex 4 and 5 significantly increases the caspase activity by 2.5 and 4 fold respectively, in T98G cells at 2 μM concentration. Whereas 4 and 5 drastically increases caspase activity by 6.3 and 9 fold respectively, at higher dose (8 μM).
Figure 10.
Effects of complex 4 and 5 on the mitochondrial membrane potential in T98G glioma cells. Plot shows JC-1 reagent red fluorescence intensity curve of cells after treatment with complexes. The horizontal axis shows the red fluorescence intensity (PE-A), and the vertical axis indicates the cell count. The dark blue curve indicates the untreated negative control and black curve indicates positive control cell population is treated by proton ionophore carbonyl cyanide 3-chlorophenylhydrazone (cccp). A curve shift from the negative control curve to the cccp treated curve indicates a loss of mitochondrial membrane potential.
Figure 11.
Effect of complex 4 and 5 on mitochondrial membrane potential (MMP) and caspase activity in T98G glioma cells population at 24 h following treatment. (A) The relative number value of JC-1 red fluorescence to negative control was quantitated by flow cytometric analysis with JC-1 reagent for detection of MMP change in T98G cells. (B) Detection of apoptosis executioner caspase activity in T98G treated cells. Induction of apoptotic cell death can justify by measuring caspase-3/7 activity using the Promega Caspase-3/7Glo assay kit, and detection of luminescence is directly proportional to the level of caspase inside the cell.
Fluorescence detection and microscopy
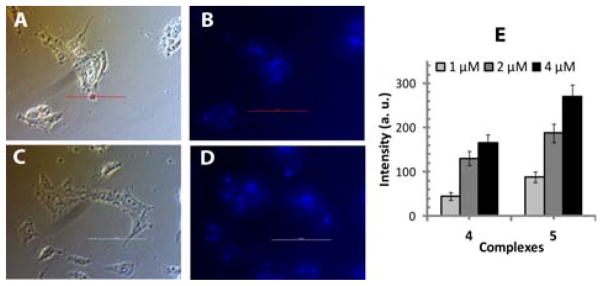
The uptake of 4 and 5 was also studied by quantification of the characteristic fluorescence of ethynyl pyridine amide ligand in cells exposed to the complexes at concentrations 1–4 μM. The results in Figure 12 revealed that T98G cells presented a good cellular uptake profile. These results were checked by fluorescence microscopy and shown in Figure 12 and Figure S4 (supporting information).
Figure 12.
Fluorescence of 4 and 5 complexes detected in T98G brain tumor cells at 24 h following treatment. Fluorescence microscopy (at 40x magnification) of T98G cells incubated with 2μM of 4 and 5: (A) bright field image with 4, (B) fluorescence image with 4, and (C) bright field image with 5, (D) fluorescence image with 5. (E) Fluorescence intensity of 4 and 5 complexes detected in T98G cells at various concentrations.
These results show that cells exposed to complex 5 had the highest fluorescence intensity. The intensity of those exposed to complex 4 were slightly lower fluorescence than the cells exposed to complex 5, but were significant when compared to untreated controls. The uptake of free ligand taken as positive control was also studied by the quantification of the characteristic fluorescence in T98G tumour cells. Interestingly, very weak fluorescence was detected in T98G tumour cells during their incubation with free ligand, indicating a lower uptake of ligand 1 by the cells, compared to the complexes (4 and 5).
Conclusion
In conclusion, we report the preparation of two new, large, self-assembled HBMCs via self-assembly of amide-based dipyridyl ligands and two different metal (Pd and Pt) acceptors. Both new metallacycles, 4 and 5, were characterized by multinuclear (1H, 13C, and 31P) NMR spectroscopy, elemental analysis, UV-Vis spectroscopy, and ESI-MS analysis. The solid-state structure of 4 was determined by molecular mechanics force field calculations, confirming the metallacyclic structure that has been assigned to these complexes.
The new self-assembled HBMCs were further screened for in vitro anticancer activity against T98G (brain tumor), KB (head and neck cancer), and SNU-80 (thyroid cancer) human cancer cell lines, and compared with the reference drug cisplatin. Both complexes found to be more efficacious against cancer cells and less toxic to normal human HEK-293 cells at effective concentrations (0.5–16 μM). Complex 5 presented the best overall parameters including: (a) high activity against the T98G brain cancer cell line and (b) low cytotoxicity on HEK-293 cells at effective concentrations. Taken together, according to the cell growth inhibitory experiments, apoptosis, MMP change, and caspase assay results, we can conclude that treatments with complexes 4 and 5 were both have a significant inhibitory effect on cell growth. The decreased viability of T98G cells strongly suggests that genotoxicity and oxidative stress was induced by the compounds and resulted in cell death or apoptosis. Cellular uptake and fluorescence microscopic study revealed that the complexes were able to carry and deliver ligand molecule intracellularly following uptake by cells. Intracellular uptake of complex 5 was slightly higher as compared to the complex 4. The importance of such work lies in the possibility that next-generation metal complexes might be efficacious as anticancer agents. However, further investigations related to identifying the structure and activity of these molecules, as well as their stability under physiological conditions, will be beneficial and may result in the design of more potent anticancer agents for therapeutic use.
Materials and methods
All chemicals used in this work were purchased from commercial sources and used without further purification. The starting cis-[(dppf)M(OTf)2] (M = Pd, Pt)[23] acceptors and ligands[24] were prepared as previously described. The 1H, 13C, and 31P {H} NMR spectra were recorded on a Bruker 300 MHz NMR spectrometer. 31P {H} chemical shifts are reported relative to an external, unlocked sample of H3PO4 (δ = 0.0 ppm). Mass spectra were recorded on a Micromass Quattro II triple-quadrupole mass spectrometer using electrospray ionization (ESI) with a MassLynx operating system. Elemental analyses were performed using the Elemental GmbH Vario EL-3 analyzer. Absorption spectra were recorded using a Cary 100 Conc UV-Visible spectrophotometer.
Reagents
Trypan blue, crystal violet, MTT and Hoechst-33342 were obtained from the Sigma Chemical Co., Korea. Phosphate buffered saline (PBS), Dulbecco’s modified eagle’s medium (DMEM), and fetal bovine serum (FBS) and trypsin-EDTA were obtained from Hyclone, Korea. H2DCFDA was obtained from Invitrogen, Molecular Probes, Korea. All other chemicals used in the present study were of analytical grade and obtained from Sigma-Aldrich, Korea.
Human Cell Culture
T98G, KB, SNU-80 and HEK-293 cells used in the present studies were purchased from the Korean Cell Line Bank (KCLB), Seoul. We cultured these cell lines in 75-cm2 culture flasks (SPL, Korea) using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10.0% fetal bovine serum, 1% nonessential amino acids, 1.0% glutamine, penicillin (100 IU/mL), and streptomycin (100 μg/mL) (all from Hyclone) or in accordance with distributor instructions. All cultures were maintained at 37°C, 95% relative humidity, and 5.0% CO2.
in vitro cell growth inhibition assay (MTT assay)
Cells were seeded in 96-well plates at a concentration of 1 × 105 cells/well in 100 μL of complete media and incubated for 24 h at 37°C in a 5.0% CO2 atmosphere to allow for cell adhesion. Stock solutions (2M) of the complexes made in a solution of DMSO: water (1:3), and then further diluted up to 0.125 μM in an incomplete media for treatment of cell lines. A calculated concentration of each complex was added to a 100 μL solution of fresh medium and distributed into wells, allowing for final concentrations of 300-0.07 μM. All assays were performed in three independent sets of duplicate tests. A negative control group, to which no drugs was applied, and a positive control group, treated with cisplatin, was also run in each assay. Following 24 h of cell exposure to drugs, each well was carefully rinsed with 200 μL PBS buffer. Cytotoxicity was assessed using 20 μL MTT solution (5 mg/ml) along with 100 μL of fresh complete media were added to each well and plates were incubated for 3 h. Following incubation, the medium was removed and the purple formazan precipitate in each well was sterilized in 100 μL DMSO. Absorbance was measured using a Biotek Synergy HT microplate reader at 540 nm and percentage (%) viability was calculated, which is directly proportional to the number of metabolically active cells:
Results, expressed as inhibitory concentration 50 (IC50), were obtained by fitting values to trend dose-response curves using the formulas provided in Microsoft Excel.
Growth Kinetics
T98G Cells were seeded at 10000 cells/cm2 on cell culture plates and their proliferation kinetics were studied at different incubation times (24, 48, and 72 h) after synthesized complex treatment. Cells were trypsinized and the total cells per plate were counted by trypan blue exclusion method microscopically.[25]
Clonogenic assay
A colony formation assay essentially tests every cell in the population for its ability to undergo “unlimited” division.[26,27] 150–400 (depending on the treatment) cells will be plated 10–12 h before treatment in DMEM at 37°C. Cultured cells were also treated with doses between 1 and 16 μM of the synthesized complexes. After treatment, cells were incubated in the dark in a humidified, 5.0% CO2 atmosphere at 37°C for 8–10 days to allow colony formation. Colonies were fixed with methanol and stained with 1.0% crystal violet. Colonies of more than 50 cells were counted and the surviving fraction (SF) of each colony was calculated. Clonogenic survival curves were constructed from three independent experiments by least-squares regression fitting the level of average survival.
Assessment of the stability of the complexes
For evaluation of the stability of 4 and 5 synthesized complexes, stability was checked from 0 h to 50 h in both solutions. Aqueous solutions with either DMSO (6 μM) or DMEM media (6 μM) were used to test the stability of the complexes. The stability of complexes was evaluated by MTT assay to check metabolic growth inhibition in T98G tumor cells.
ATP measurement
All multicellular organisms utilize ATP as a means for storage metabolic energy. Therefore, the quantitation of ATP signals as a hallmark to detect metabolically active cells. For that reason we checked intracellular ATP level in 4 and 5 complex treated cells. We used different concentrations of complexes to perform this experiment. ATP was measured using commercial kit (CellTiter-Glo® Luminescent Cell Viability Assay, Promega, Korea) and all steps of detection were performed as manufactures protocol.
Intracellular ROS detection
In order to check intracellular ROS induced by 4 and 5 complexes in T98G cells, we used 2′,7′-dichlorofluorescin diacetate (H2DCFDA; Molecular Probes, USA) fluorochrome probe. Upon oxidation, non-fluorescent compound DCFH-DA generates the fluorescent compound dichlorofluorescein (DCF). Thus, DCF fluorescence represents the rate and quantity of ROS produced. Briefly, after treatment with complexes, T98G cells were incubated in fresh media with 10 μM H2DCFDA at 37°C for 30 min, washed twice with PBS, and then measured for fluorescence using a plate reader (Biotek, Techan, USA) with excitation at 485 nm and emission at 528 nm.
FACS analysis of annexin V and PI staining
Flow cytometry was used to confirm levels of apoptosis in T98G cells treated with complexes 4 and 5. For analysis of treatment-induced cell death, annexin V-FITC/PI staining was performed followed by FACS analysis. Cells were seeded and treated with 0.05, 1, and 2 μM concentrations of complexes. Untreated cells and cells treated with cisplatin (to trigger apoptosis) were used as a negative control and positive control, respectively. Briefly, 24 h after treatment, cells were collected and subjected to annexin V-FITC/PI staining using the EzWay Annexin V-FITC apoptosis detection kit, following all steps in the protocol provided by the manufacturer.
Mitochondrial membrane potential detection
Mitochondria have an crucial role in cell apoptosis induced by many factors, and the decline of mitochondrial membrane potential (ΔΨm) is an earlier landmark/event of the apoptosis.[28] JC-1 is an sensitive marker for the detection of mitochondrial membrane potential in apoptotic cells. JC-1 is a lipophilic cationic dye. It exists in monomer, lower concentration show green fluorescence (depolarized mitochondrial membrane) in cells. However, at higher concentrations, the dye forms J-aggregates that exhibit a broad excitation spectrum and an emission maximum at ~590 nm, cells show red fluorescence (normal polarized mitochondria). To observe the effect of 4 and 5 complexes on mitochondrial membrane, JC-1 staining was performed on complex treated T98G cells with MitoProbe JC-1 Assay Kit (Invitrogen, molecular probes) according to the manufacturer’s instructions. For positive MMP depolarization, we used a proton ionophore Carbonyl cyanide 3-chlorophenylhydrazone (cccp), which degrade the membrane potential quickly.
Casapse 3/7 activity measurement
Caspases, a family of cysteine proteases, are the central regulators of apoptosis. One of the most well studied members of this cysteine protease family includes executioner caspase-3 and caspase-7, which plays a key role in cell apoptosis and differentiation.[29] In order to measure apoptosis, the Caspase-Glo 3/7® Assay Kit (Promega, Korea) was used. 100 μL Caspase-Glo® 3/7 reagent was added to each 100 μL of complexes treated and control cells and incubated at room temperature. Luminescence was recorded of each sample in a plate using luminometer (Biotek, Techan, USA).
Evaluation of cellular uptake and microscopy of complexes
Cells were seeded in 96-well plates (SPL, Korea) and incubated 12 h for cell adhesion. Then cells were exposed to 1–4 μM concentrations of 4 and 5 complexes at 37°C for 48 h in complete culture medium. After washing with PBS, the supernatants were replaced with fresh medium and the complex treated cell population was evaluated by fluorescence microplate reader (Biotek, Techan, USA) with excitation and emission filters set at 330 and 485 nm, respectively. For microscopy, cells were grown on glass slides in complete culture medium and exposed overnight to the 4 and 5 using 2 μM concentrations. Slides were washed with PBS and examined under a fluorescence microscope (Ti-U, Nikon) with filters set MBE41300 C-FL consisting of excitation filter Ex340-380 and barrier filter BA435-485.
Synthesis of hetro-bimetallacycle 4
Ligand 1 (0.010g, 0.020 mmol) and Pd(dppt)2(OTf)2 (0.020 g, 0.020 mmol) acceptor 2 were suspended in CH2Cl2/CH3NO2 (1:1, 2.0 mL) and the resulting mixture was stirred for 5–6 h at room temperature. The reaction mixture was filtered, the solvent was removed under reduced pressure, and the product was washed with diethyl ether. The crude product obtained was redissolved in nitromethane and subjected to vapor diffusion of diethyl ether. This resulted in a highly red crystalline product within one day (93.0%, 0.028 mg). Elemental analysis calculated (%) for C138H97F12Fe2N10O16P4Pd2S4: C 56.07, H 3.31, N 4.74; found: C 56.88, H 3.42, N 4.60; MS (ESI) calculated for [M – 3OTf]3+ m/z 836.5, found 836.4; calculated for [M – 4OTf]4+ m/z 590.1, found 590.1; 1H NMR (300 MHz, [D3] nitromethane, δ, ppm): 10.17 (s, 4H, CONH), 8.46 (d, 8H, Ha), 8.43-8.24 (m, 10H, He/f), 8.03-7.94(m, 32H, Ph), 7.78(m, 8H, Hb), 7.71-7.67(m, 8H, Ph) 7.55 (d, 8H, Hd), 7.20 (d, 8H, Hd), 4.83 (m, 8H, cp), 4.76 (m, 8H, cp); 13C NMR (300 MHz, [D3] nitromethane, δ, ppm): 164.13 (C=O), 151.01, 149.36, 141.39, 135.50, 134.60, 131.26, 129.14, 128.38, 127.66, 124.29, 120.05, 78.40, 77.42, 70.30, 69.40, 30.78.
Synthesis of hetro-bimetallacycle 5
Synthesis was performed in a manner similar to HBMC 4, except that the Pd(dppt)2(OTf)2 acceptor 3 was used and the product was isolated as a yellow crystalline solid with 91.0% yield. Elemental analysis calculated (%) for C138H97F12Fe2N10O16P4Pt2S4: C 56.07, H 3.31, N 4.74; found: C 57.01, H 3.39, N 4.81; MS (ESI) calculated for [M-3OTf]3+ m/z 895.5, found 895.4; calculated for [M – 4OTf]4+ m/z 634.4, found 634.4; 1H NMR (300 MHz, [D3] nitromethane, δ, ppm): 10.48 (s, 4H, CONH), 8.29 (m, 8H, Ha), 7.67 (m, 8H, Hb), 7.77 (m, 8H, Hc), 8.15 (m, 2H, Hd), 8.03-7.98 (m, 40H, Ph), 5.81 (m, 8H, cp), 5.79 (m, 8H, cp); 13C NMR (300 MHz, [D3] nitromethane, δ, ppm): 165.11 (C=O), 152.02, 148.36, 141.38, 135.57, 133.78, 130.92, 129.14, 128.48, 127.67, 124.54, 120.11, 77.41, 78.49, 70.34, 69.77, 31.18.
Supplementary Material
Figure 6.
Geometry-optimized structure of 4 obtained from a molecular mechanics force field calculation. (a) Schematic diagram showing the two Pd2+ ions (green) coordinated by four pyridine N (blue). (b) Side view shown as a space-filling model.
Acknowledgments
This work was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Science, ICT and Future Planning (NRF-2013R1A1A2006859). We also thank the Priority Research Centers program (2009-0093818) through the NRF for financial support. NKK and EHC express gratitude for support from the National Research Foundation of Korea (NRF) funded by the Korea government (MSIP) (NRF-2010-0027963) and Kwangwoon University in 2014. PJS thanks the NIH for financial support.
Footnotes
Supporting information (NMR data, graph of apoptosis, microscopic fluorescence image) for this article is available on the WWW under http://www.chemeurj.org/
References
- 1.a) Lehn J-M. Supramolecular Chemistry, concepts and perspectiVes. VCH; New York: 1995. [Google Scholar]; b) Steed JW, Atwood JL. Supramolecular Chemistry. VCH; New York: 2000. [Google Scholar]; c) Leininger S, Olenyuk B, Stang PJ. Chem Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; d) Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; e) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc Chem Res. 2005;38:349. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; f) Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc Chem Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Raymond KN. Nature. 2009;460:585. [Google Scholar]; h) Pluth MD, Bergman RG, Raymond KN. Acc Chem Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; i) Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Chem Soc Rev. 2009;38:1450. doi: 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]; j) Pluth MD, Bergman RG, Raymond KN. J Am Chem Soc. 2008;130:6362. doi: 10.1021/ja076691h. [DOI] [PubMed] [Google Scholar]; k) Klosterman JK, Yamauchi Y, Fujita M. Chem Soc Rev. 2009;38:1714. doi: 10.1039/b901261n. [DOI] [PubMed] [Google Scholar]; l) Severin K. Chem Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; m) Mishra A, Gupta R. Dalton Trans. 2014;14:6768. doi: 10.1039/c4dt00277f. [DOI] [PubMed] [Google Scholar]
- 2.a) Northrop BH, Zheng YR, Chi KW, Stang PJ. Acc Chem Res. 2009;42:1554. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zheng YR, Yang HB, Northrop BH, Ghosh K, Stang PJ. Inorg Chem. 2008;47:4706. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]; c) Zheng YR, Yang HB, Ghosh K, Zhao L, Stang PJ. Chem Eur J. 2009;15:7203. doi: 10.1002/chem.200900230. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Seidel SR, Stang PJ. Acc Chem Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; e) Ghosh S, Mukherjee PS. Inorg Chem. 2009;48:2605. doi: 10.1021/ic802254f. [DOI] [PubMed] [Google Scholar]; f) Shanmugaraju S, Bar AK, Chi KW, Mukherjee PS. Organometallics. 2010;29:2971. [Google Scholar]; g) Mishra A, Dubey A, Min JW, Kim H, Stang PJ, Chi KW. 2014;50:7542. doi: 10.1039/c4cc01991a. [DOI] [PubMed] [Google Scholar]
- 3.a) Stang PJ, Olenyuk B. Acc Chem Res. 1997;30:502. [Google Scholar]; b) Northrop BH, Yang HB, Stang PJ. Chem Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ghosh S, Mukherjee PS. Inorg Chem. 2009;48:549. doi: 10.1021/ic801381p. [DOI] [PubMed] [Google Scholar]; d) Bar AK, Chakrabarty R, Mostafa G, Mukherjee PS. Angew Chem, Int Ed. 2008;47:8455. doi: 10.1002/anie.200803543. [DOI] [PubMed] [Google Scholar]; e) Ghosh S, Mukherjee PS. J Org Chem. 2006;71:8412. doi: 10.1021/jo061311g. [DOI] [PubMed] [Google Scholar]; f) Shanmugaraju S, Bar AK, Chi K-W, Mukherjee PS. Organometallics. 2010;29:2971. [Google Scholar]
- 4.a) Barbour LJ, Orr GW, Atwood JL. Nature. 1998:393. [Google Scholar]; b) Qin Z, Jennings MC, Puddephatt RJ. Chem Commun. 2001:2676. [Google Scholar]; c) Qin Z, Jennings MC, Puddephatt RJ. Chem Commun. 2002:354. doi: 10.1039/b110307e. [DOI] [PubMed] [Google Scholar]; d) Qin Z, Jennings MC, Puddephatt RJ. Inorg Chem. 2003;42:1956. doi: 10.1021/ic020322z. [DOI] [PubMed] [Google Scholar]; e) Mishra A, Ali A, Upreti S, Whittingham MS, Gupta R. Inorg Chem. 2009;48:5234. doi: 10.1021/ic900223f. [DOI] [PubMed] [Google Scholar]; f) Mishra A, Ali A, Upreti S, Gupta R. Inorg Chem. 2008;47:154. doi: 10.1021/ic7016424. [DOI] [PubMed] [Google Scholar]; g) Mishra A, Kaushik NK, Verma AK, Gupta R. Eur J Med Chem. 2008;43:2189. doi: 10.1016/j.ejmech.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 5.a) Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J Am Chem Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]; b) Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:371. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; c) Oin Z, Jennings MC, Puddephatt RJ. Chem Commun. 2001:2676. [Google Scholar]; d) Leininger S, Fan J, Schmitz M, Stang PJ. Proc Nalt Acad Sci USA. 2000;97:1380. doi: 10.1073/pnas.030264697. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mishra A, Kim H, Lee SC, Min JW, Lee MH, Chi KW. Inorg Chim Acta. 2013;405:77. [Google Scholar]; f) Amijs CHM, van Klink GPM, Koten G. Dalton Trans. 2006:308. doi: 10.1039/b505354d. [DOI] [PubMed] [Google Scholar]; g) Yamaguchi T, Tashiro S, Tominaga M, Kawano M, Ozeki T, Fujita M. Chem Asian J. 2007;2:468. doi: 10.1002/asia.200600429. [DOI] [PubMed] [Google Scholar]; h) Tashiro S, Tominaga M, Yamaguchi Y, Kato K, Fujita M. Angew Chem Int Ed. 2006;45:241. doi: 10.1002/anie.200502802. [DOI] [PubMed] [Google Scholar]; i) Goshe AJ, Steele IM, Ceccarelli C, Rheingold AL, Bosnich B. Proc Natl Acad Sci USA. 2002;99:4823. doi: 10.1073/pnas.052587499. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Fiedler D, van Halbeek H, Bergman RG, Raymond KN. J Am Chem Soc. 2006;128:10240. doi: 10.1021/ja062329b. [DOI] [PubMed] [Google Scholar]; k) Shin RYC, Tan GK, Koh LL, Vittal JJ, Goh LY, Webster RD. Organometallics. 2005;24:539. [Google Scholar]; l) Mondal KC, Song Y, Mukherjee PS. Inorg Chem. 2007;46:9736. doi: 10.1021/ic701018x. [DOI] [PubMed] [Google Scholar]; m) Ghosh S, Mukherjee PS. Dalton Trans. 2007:2542. doi: 10.1039/b617513a. [DOI] [PubMed] [Google Scholar]; n) Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]
- 6.a) Schilter D, Clegg JK, Harding MM, Rendina LM. Dalton Trans. 2010;39:239. doi: 10.1039/b916579g. [DOI] [PubMed] [Google Scholar]; b) Schilter D, Urathamakul T, Beck JL, Hannon MJ, Rendina LM. Dalton Trans. 2010;39:11263. doi: 10.1039/c0dt00754d. [DOI] [PubMed] [Google Scholar]; c) Barry NPE, Abd Karim NH, Vilar R, Therrien B. Dalton Trans. 2009:10717. doi: 10.1039/b913642h. [DOI] [PubMed] [Google Scholar]; d) Kieltyka R, Englebienne P, Fakhoury J, Atutexier C, Moitessier N, Sleiman HF. J Am Chem Soc. 2008;130:10040. doi: 10.1021/ja8014023. [DOI] [PubMed] [Google Scholar]
- 7.a) Cardo L, Sadovnikova V, Phongtongpasuk S, Hodges NJ, Hannon MJ. Chem Commun. 2011;47:6575. doi: 10.1039/c1cc11356a. [DOI] [PubMed] [Google Scholar]; b) Ducani C, Leczkowska A, Hodges NJ, Hannon MJ. Angew Chem Int Ed. 2010;49:8942. doi: 10.1002/anie.201004471. [DOI] [PubMed] [Google Scholar]; c) Boer DR, Kerckhoffs JMCA, Parajo Y, Pascu M, Uson I, Lincoln P, Hannon MJ, Coll M. Angew Chem Int Ed. 2010;49:2336. doi: 10.1002/anie.200906742. [DOI] [PubMed] [Google Scholar]; d) Cardo L, Hannon MJ. Inorg Chim Acta. 2009;362:784. [Google Scholar]; e) Oleksi A, Blanco AG, Boer R, Uson I, Aymam J, Rodger A, Hannon MJ, Coll M. Angew Chem Int Ed. 2006;45:1227. doi: 10.1002/anie.200503822. [DOI] [PubMed] [Google Scholar]; f) Oleksi A, Blanco AG, Boer R, Uson I, Aymami J, Rodger A, Hannon MJ, Coll M. Angew Chem Int Ed. 2006;45:1227. doi: 10.1002/anie.200503822. [DOI] [PubMed] [Google Scholar]; g) Uerpmann C, Malina J, Pascu M, Clarkson GJ, Moreno V, Rodger A, Grandas A, Hannon MJ. Chem Eur J. 2005;11:1750. doi: 10.1002/chem.200401054. [DOI] [PubMed] [Google Scholar]; h) Moldrheim E, Hannon MJ, Meistermann I, Rodger A, Sletten E. J Biol Inorg Chem. 2002;7:770. doi: 10.1007/s00775-002-0354-2. [DOI] [PubMed] [Google Scholar]
- 8.a) Mishra A, Lee S, Kim H, Cook TR, Stang PJ, Chi KW. Chem Asian J. 2012;7:2592. doi: 10.1002/asia.201200488. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mishra A, Vajpayee V, Kim H, Lee MH, Jung H, Wang M, Stang PJ, Chi KW. Dalton Trans. 2012;41:1195. doi: 10.1039/c1dt11612f. [DOI] [PubMed] [Google Scholar]; c) Mishra A, Jung H, Lee MH, Lah MS, Chi KW. Inorg Chem. 2013;52:8573. doi: 10.1021/ic401685s. [DOI] [PubMed] [Google Scholar]; d) Mishra A, Ravikumar S, Hong SH, Kim H, Vajpayee V, Lee HW, Ahn BC, Wang M, Stang PJ, Chi KW. Organometallics. 2011;30:6343. doi: 10.1021/om200802v. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Barry NPE, Edafe F, Therrien B. Dalton Trans. 2011;40:7172. doi: 10.1039/c1dt10489f. [DOI] [PubMed] [Google Scholar]; f) Barry NPE, Zava O, Furrer J, Dyson PJ, Therrien B. Dalton Trans. 2010;39:5272. doi: 10.1039/c001521k. [DOI] [PubMed] [Google Scholar]; g) Barry NPE, Edafe F, Dyson PJ, Therrien B. Dalton Trans. 2010;39:2816. doi: 10.1039/b925015h. [DOI] [PubMed] [Google Scholar]; h) Therrien B. Eur J Inorg Chem. 2009:2445. [Google Scholar]
- 9.a) Zava O, Mattsson J, Therrien B, Dyson PJ. Chem Eur J. 2010;16:1428. doi: 10.1002/chem.200903216. [DOI] [PubMed] [Google Scholar]; b) Hartinger CG, Dyson PJ. Chem Soc Rev. 2009;38:391. doi: 10.1039/b707077m. [DOI] [PubMed] [Google Scholar]; c) Hambley TW. Dalton Trans. 2007:4929. doi: 10.1039/b706075k. [DOI] [PubMed] [Google Scholar]; d) Orvig C, Abrams MJ. Chem Rev. 1999;99:2201. doi: 10.1021/cr980419w. [DOI] [PubMed] [Google Scholar]; e) Fish RH, Jaouen G. Organometallics. 2003;22:2166. [Google Scholar]; f) Kostova I. Recent Pat. Anti-Cancer Drug Discovery. 2006;1:1. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]; g) Farver O. Textbook of Drug Design and Discovery. 3. Taylor & Francis Ltd; London, UK: 2002. p. 364. [Google Scholar]
- 10.Lewis JEM, Gavey EL, Cameron SA, Crowley JD. Chem Sci. 2012;3:778. [Google Scholar]
- 11.a) Mishra A, Kang SC, Chi KW. Eur J Inorg Chem. 2013:5222. [Google Scholar]; b) Schatzschneider U, Metzler-Nolte N. Angew Chem Int Ed. 2006;45:1504. doi: 10.1002/anie.200504604. [DOI] [PubMed] [Google Scholar]
- 12.Melchart M, Sadler PJ. Bioorganometallics. Wiley-VCH; Weinheim: 2006. p. 39. [Google Scholar]
- 13.a) Reedijk J. Proc Natl Acad Sci. 2003;100:3611. doi: 10.1073/pnas.0737293100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guo Z, Sadler PJ. Angew Chem, Int Ed. 1999;38:1512. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; c) Reedijk J. Plat Met Rev. 2008;52:2. [Google Scholar]
- 14.Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew Chem, Int Ed. 2008;47:3773. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]
- 15.a) Mishra A, Jung H, Park JW, Kim HK, Kim H, Stang PJ, Chi KW. Organometallics. 2012;31:3519. doi: 10.1021/om2012826. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dubey A, Min JW, Koo HJ, Kim H, Cook TR, Kang SC, Stang PJ, Chi KW. Chem-Eur J. 2013;19:11622. doi: 10.1002/chem.201300870. [DOI] [PubMed] [Google Scholar]; c) Mishra A, Jeong YJ, Jo JH, Kang SC, Kim H, Chi KW. Organometallics. 2014;33:1144. [Google Scholar]; d) Mishra A, Jeong YJ, Jo JH, Kang SC, Lah MS, Chi KW. ChemBioChem. 2014;15:695. doi: 10.1002/cbic.201300688. [DOI] [PubMed] [Google Scholar]
- 16.Rappe AK, Casewit CJ, Colwell KS, Goddard WA, Skiff WM. J Am Chem Soc. 1992;114:10024. [Google Scholar]
- 17.Frisch MJ, Trunk GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision C.01. Gaussian, Inc; Wallingford, CT: 2009. [Google Scholar]
- 18.a) Barry NPE, Sadler PJ. Chem Soc Rev. 2012;41:3264. doi: 10.1039/c2cs15300a. [DOI] [PubMed] [Google Scholar]; b) Kaushik NK, Mishra A, Ali A, Adhikari JS, Verma AK, Gupta R. J Biol Inorg Chem. 2012;17:1217. doi: 10.1007/s00775-012-0937-5. [DOI] [PubMed] [Google Scholar]; c) Lippard SJ. J Am Chem Soc. 2010;132:14689. doi: 10.1021/ja108523h. [DOI] [PubMed] [Google Scholar]; d) Bergamo A, Sava G. Dalton Trans. 2011;40:7817. doi: 10.1039/c0dt01816c. [DOI] [PubMed] [Google Scholar]; e) Levina A, Mitra A, Lay PA. Metallomics. 2009;1:458. doi: 10.1039/b904071d. [DOI] [PubMed] [Google Scholar]; f) Atilla-Gokcumen GE, Di Costanzo L, Meggers E. J Biol Inorg Chem. 2011;16:45. doi: 10.1007/s00775-010-0699-x. [DOI] [PubMed] [Google Scholar]; g) Wahab R, Kaushik NK, Verma AK, Mishra A, Hwang IH, Yang YB, Shin HS, Kim YS. J Biol Inorg Chem. 2011;16:431. doi: 10.1007/s00775-010-0740-0. [DOI] [PubMed] [Google Scholar]; h) Hannon MJ. Chem Soc Rev. 2007;36:280. doi: 10.1039/b606046n. [DOI] [PubMed] [Google Scholar]
- 19.Paul LEH, Therrien B, Furrer J. Inorg Chem. 2012;51:1057. doi: 10.1021/ic2021935. [DOI] [PubMed] [Google Scholar]
- 20.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Cell Death Differ. 2009;16:3. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. J Immunol Meth. 1995;184:39. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 22.a) Green DR, Kroemer G. Science. 2004;305:626. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]; b) Green DR, Reed JC. Science. 1998;281:1309. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]; c) Gottlieb E, Armour SM, Harris MH, Thompson CB. Cell Death Differ. 2003;10:709. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]; d) Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. J Exp Med. 1995;182:367. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang PJ, Fan BJ, Arif AM. Organometallics. 1996;15:904. [Google Scholar]
- 24.Mishra A, Ravikumar S, Song Y, Prabhu NS, Hong SH, Kim H, Cheon S, Noh J, Chi K-W. Dalton Trans. 2014;43:6032. doi: 10.1039/c3dt53186d. [DOI] [PubMed] [Google Scholar]
- 25.Kaushik NK, Kim HS, Chae YJ, Lee YN, Kwon GC, Choi EH, Kim IT. Molecules. 2012;17:11456. doi: 10.3390/molecules171011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaushik NK, Attri P, Kaushik N, Choi EH. Molecules. 2012;17:13727. doi: 10.3390/molecules171213727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaushik NK, Uhm HS, Choi EH. Appl Phys Lett. 2012;100:084102. [Google Scholar]
- 28.Liu C, Yin L, Chen J. Tumour Biol. 2014;35:1791. doi: 10.1007/s13277-013-1238-5. [DOI] [PubMed] [Google Scholar]
- 29.Vickers CJ, González-Páez GE, Wolan DW. ACS Chemical Biology. 2013;8:1558. doi: 10.1021/cb400209w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.