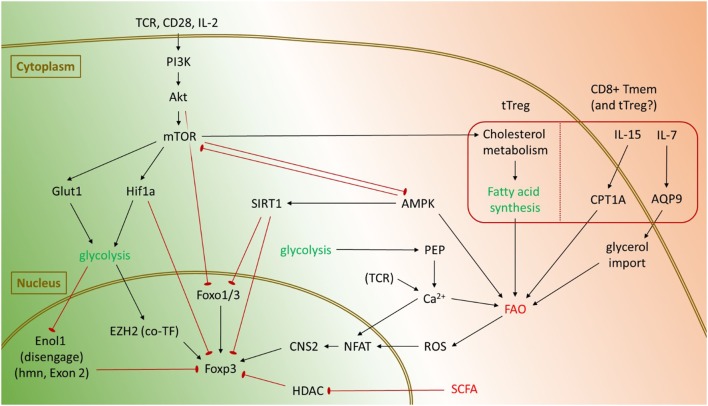
Figure 2.
Pathways promoting glycolysis and fatty acid oxidation (FAO) in regulatory T cells (Tregs), and known mechanisms affecting Foxp3. Glycolysis is primarily activated in Tregs through mTOR and tends to suppress Foxp3 expression and Treg lineage stability. Activation of the PI(3)K/Akt/mTOR signaling axis inhibits Foxo transcription factors and promotes activation of Hif-1α, which can directly target Foxp3 for degradation. However, under certain conditions, glycolysis also promotes Foxp3 expression. By disengaging Enolase 1 from its nuclear role, glycolysis enables expression of the Foxp3-E2 splice isoform in humans. Glycolysis also represses microRNAs such as miR-101 and miR-26a to enable expression of EZH2, which is a cotranscription factor for Foxp3. Tregs generally rely upon FAO for their metabolic needs. In the gut, short-chain fatty acids (SCFA) inhibit histone deacetylases (HDACs) to promote Foxp3 expression and conversion of naïve CD4+ T cells into pTregs. Under certain conditions, FAO may also impinge upon Treg lineage stability. Sirt1 may repress Foxp3, either through direct deacetylation of Foxp3 or by targeting Foxo transcription factors. In CD8+ memory T cells, cytokines such as IL-7 and IL-15 promote uptake of fatty acid precursors and increased FAO, respectively. It remains to be seen whether similar processes occur in Tregs as well. Both glycolysis and FAO can also promote Foxp3 expression through an NFAT-dependent mechanism.