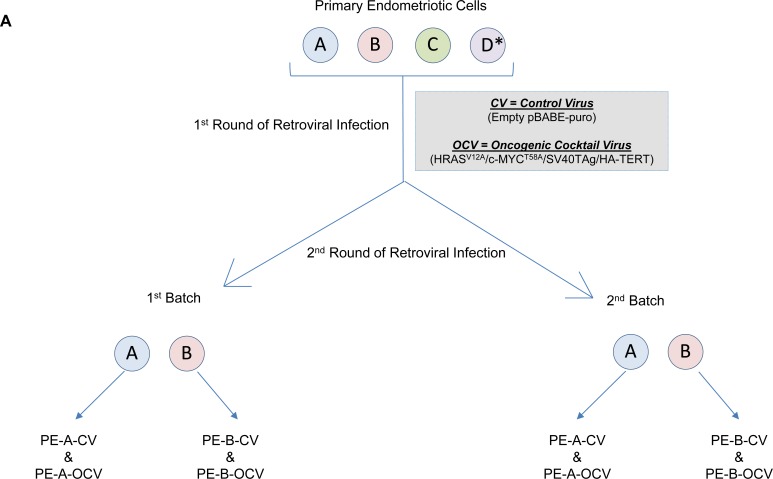
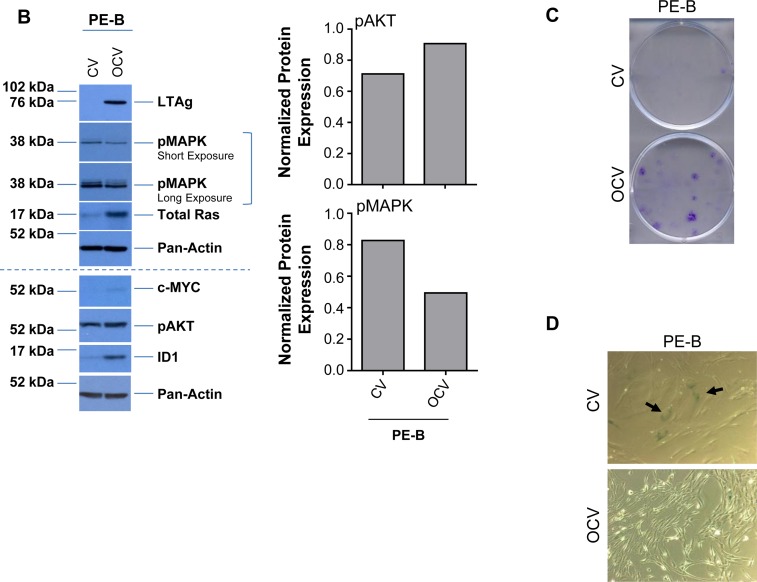
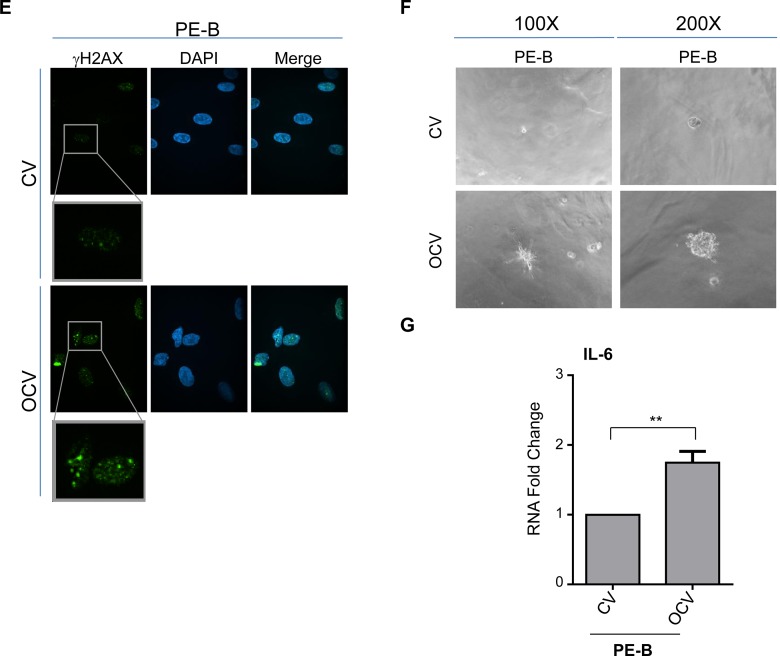
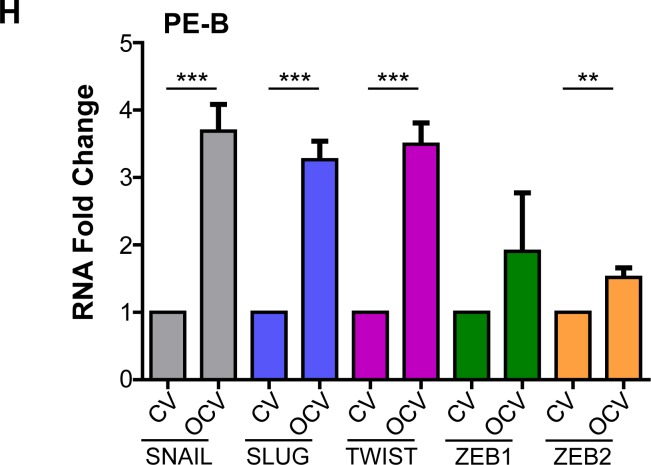
Figure 1. Transformation of human primary endometriotic cells.
(A) Schematic depicting the overall strategy involving retroviral infections (with control virus (CV) or oncogenic cocktail virus (OCV: comprised of HRASV12A, c-MYCT58A, SV40 LTAg, and HA-hTERT)) to generate transformed endometriotic from primary cells (PE-A, PE-B, PE-C, and PE-D; * refers to life-extended PE-D cells with SV40 LTAg) isolated from endometriotic lesions. Two batches of transformed endometriotic cells were successfully obtained using PE-A and PE-B primary cells. The first batch of retrovirally infected cells (PE-B-CV and PE-B-OCV) were utilized to: (B) obtain cell lysates for western blotting with the indicated antibodies (left panel). The dotted line specifies re-run samples to avoid the possibility of detecting overlapping bands of similar molecular weights. Densitometric analyses for pAKT and pMAPK are shown in the right panels; (C) perform colony formation assay and images were captured following 14 days in culture (representative images are shown, three independent experiments were conducted); (D) perform β-galactosidase staining and images were captured at 100× magnification (representative images are shown, three independent experiments were conducted); and (E) assess DNA damage via γH2AX immunofluorescence staining (representative images shown were captured at 63× magnification and the images of nuclei were enlarged and cropped using PowerPoint to focus on the DNA damage foci). The second batch of retrovirally infected cells (PE-B-CV and PE-B-OCV) were utilized to: (F) assess the in vitro tumorigenic potential (by 3-dimensional morphogenesis assay in Matrigel). Representative images (from four independent experiments) were captured at 100× (left) and 200× (right) magnification; (G) to measure IL-6 transcript levels via real-time PCR. Three independent experiments were performed; and (H) assess transcript levels for genes in the EMT pathway via real-time PCR (three independent experiments were performed).