Abstract
Background: The evidence base to support palliative care clinical practice is inadequate and opportunities to improve the palliative care evidence base remain despite the field's rapid growth.
Objective: To examine current NIH funding of palliative medicine research, changes since our 2013 report, and trends since our 2008 report.
Design: We sought to identify NIH funding of palliative medicine from 2011 to 2015 in two stages: (I) we searched the NIH grants database “RePorter” for grants with key words “palliative care,” “end-of-life care,” “hospice,” and “end of life” and (II) we identified palliative care researchers likely to have secured NIH funding using three strategies.
Methods: We abstracted (1) the first and last authors' names from original investigations published in major palliative medicine journals from 2013 to 2015; (2) these names from a PubMed-generated list of original articles published in major medicine, nursing, and subspecialty journals using the above key words; and (3) palliative medicine journal editorial board members and key members of palliative medicine initiatives. We crossmatched the pooled names against NIH grants funded from 2011 to 2015.
Results: The author and NIH RePorter search identified 854 and 419 grants, respectively. The 461 grants categorized as relevant to palliative medicine represented 334 unique PIs. Compared to 2006–2010, the number of NIH-funded junior career development awards nearly doubled (6.1%–10%), articles published in nonpalliative care specialty journals tripled (13%–37%), published palliative care researchers increased by 2.5-fold (839–2120), and NIH-funded original palliative medicine research articles doubled (21%–39%).
Conclusions: Despite the challenging NIH funding climate, NIH funding to palliative care remained stable. The increase in early stage career development funding, palliative care investigators, and palliative medicine research published in nonpalliative medicine journals reflects important advances to address the workforce and evidence gaps. Further support for palliative care research is still needed.
Keywords: : NIH, palliative medicine, research funding
Introduction
Palliative medicine has continued to expand to address the unmet needs of patients with serious illness and their families. The 2015 State-By-State Report Card on Access to Palliative Care in Our Nation's Hospitals revealed that 67% of U.S. hospitals with 50 or more beds now have palliative care teams, up from 63% in 2011 and 53% in 2008.1 Furthermore, models of palliative care delivery are now being developed in nonhospice and hospital settings, expanding access to those living in the community. Nevertheless, the evidence base to support the clinical practice of palliative medicine remains underdeveloped.2–4 If improvement of the care for the seriously ill, their families, and their caregivers is a priority, then federal investment in research is needed to create an adequate evidence base.
In our first report on NIH funding for palliative care research (2001–2005), we noted that more than 25% of published palliative medicine research was performed without any acknowledged extramural funding and less than one-third of published studies were supported by NIH funding.5 In our 2006–2010 report, we found modest improvements in the number of grants awarded by NIH for palliative care research and the number of NIH funding for palliative care investigators, and an almost threefold increase in the number of original research articles published compared to 2001–2005.6
Since our 2013 report, a number of new national initiatives have been expanded to stimulate and support new federally funded palliative care research. Dedicated organizations such as the National Palliative Care Research Center (NPCRC)7–9 and the National Institute for Nursing Research (NINR) (UC4-NR012584, U24-NR014637) continue funded Palliative Care Research Cooperative2,10–12 to target funding for palliative care research and support early-stage and mid-career investigators. In addition, leading nursing and physician professional organizations (e.g., American Academy of Hospice and Palliative Medicine and the Hospice and Palliative Nursing Association) have expanded efforts to disseminate research findings and support palliative care investigators.
This study was performed to continue to track NIH funding for published palliative medicine research from 2011 to 2015 to update our prior work, and to help support and guide NIH funding priorities in the field.
Methods
We undertook a two-stage process to identify NIH funding related to palliative care from 2011 to 2015. First, we identified palliative care researchers likely to have secured NIH funding. Potential NIH fundees were identified as follows: we abstracted the names of the first and senior authors' from all original investigations published in major palliative medicine journals from 2013 to 2015 (Journal of Palliative Medicine, Journal of Pain and Symptom Management, Palliative Medicine, Palliative and Supportive Care, and Journal of Hospice and Palliative Nursing). Next, we searched PubMed using the key MESH terms “palliative care,” “end-of-life care,” “hospice,” and “end of life” and abstracted the names of the first and senior authors from all articles published in major adult internal medicine journals (Annals of Internal Medicine, New England Journal of Medicine, British Medical Journal, Lancet, JAMA Internal Medicine, JAMA, and American Journal of Medicine) and relevant high-impact subspecialty journals, representing treatment of patients with serious illness (Journal of Clinical Oncology, Journal of the American Geriatrics Society, Journal of General Internal Medicine, Journals of Gerontology, American Journal of Respiratory and Critical Care Medicine, Critical Care Medicine, Thorax, Circulation, Circulation Research, Journal of the American College of Cardiology, and Health Affairs). Finally, we identified all editorial board members of palliative medicine journals, Project on Death in America Faculty Scholars,13,14 as well as the grantees and committee members from the NPCRC7 (www.npcrc.org), the American Cancer Society's Palliative Care Initiative, the Center to Advance Palliative Care (www.capc.org), and the Palliative Care Research Collaborative10 (palliativecareresearch.org).
After obtaining these names of potential funded investigators, we crossmatched this list of 2073 names against all funded NIH research projects from 2011 to 2015 by entering each name into the NIH RePORTER system.15 RePORTER is an electronic tool that allows users to search a repository of NIH-funded research projects and access publications and patents resulting from NIH funding. Next, we created a list of grants for which these abstracted names are either a PI or co-PI. We excluded those PIs from academic institutions outside the United States. From the subsequent list of grants, we abstracted the contact PI's name, the grant number, the grant title, the contact PI's institution, administering institute, activity code, fiscal year total cost (when available), and the start and end date of the grant. We did not include grants funded by other federal funding agencies such as Patient-Centered Outcomes Research Institute (PCORI), Agency for Healthcare Research and Quality (AHRQ), Veterans Administration (VA), and The Center for Medicaid and Medicare Expansion (CMMI) because funding from these agencies was not available during the reporting periods in our prior reports.
As an additional step to ensure completeness, we searched the NIH RePorter grants database for all grants with the key words “palliative care,” “end-of-life care,” “hospice,” and “end of life” and abstracted grant data detailed above for any grants not captured in our original search.
Two of the study's authors (E.E.B. and L.P.G.) independently hand reviewed all identified grants for relevance to palliative medicine, as determined by the National Consensus Project's for Quality Palliative medicine's definition.16 In the event of a disagreement, the third author (R.S.M.) also reviewed the disputed grant, and the grant was discussed until consensus was reached. Of the 854 NIH grants identified by published authors' names, the reviewers (E.E.B. and L.P.G.) agreed that 277 were relevant to palliative medicine, with 100% agreement. Of the 419 unique grants identified in the NIH Reporter key word search, which were not already identified in the author search, the reviewers (E.E.B. and L.P.G.) agreed on the categories for 180 of 184 NIH grants. For the four on which the reviewers disagreed (2%), grant topics were discussed with the third reviewer (R.S.M.) until consensus was reached.
Finally, the 461 unique grants categorized as relevant to palliative medicine were further categorized independently by each author (E.E.B. and L.P.G.) into one of the following 10 categories: (1) studies focusing on pain and physical symptom management and quality of life, (2) studies examining psychological, spiritual, and emotional symptoms, (3) studies of instrument development and measurement, (4) health services research evaluating systems of care, (5) decision making and communication studies, (6) studies of palliative medicine education and training programs, (7) studies of caregivers and families, (8) pediatrics studies, (9) career development awards, and (10) other. The reviewers (E.E.B. and L.P.G.) agreed on the categories for 429 of 461 NIH grants. For the 32 on which the reviewers disagreed (7%), grant topics were discussed with the third reviewer (R.S.M.) until consensus was reached.
Results
We identified 854 grants from our initial author search and 419 grants from the NIH RePorter search, for a total of 1273 unique grants. Of these, 461 grants were deemed relevant to palliative medicine. Three hundred thirty-four researchers were listed as unique principal investigators on these grants (mean of 1.30 grants per investigator). These 461 grants represented 0.2% of all NIH research awards.
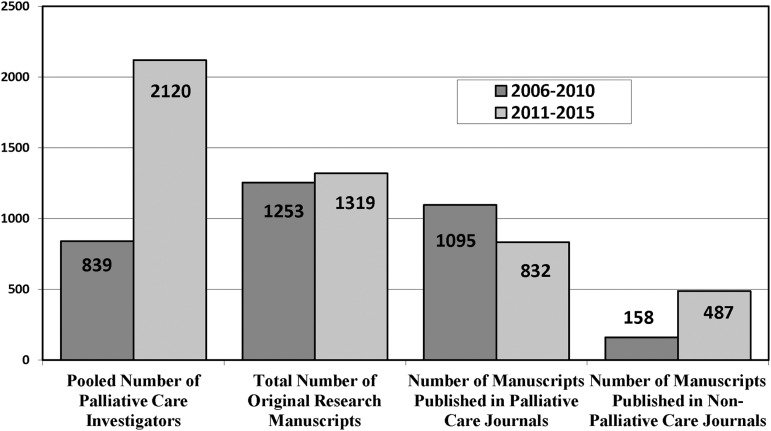
We identified 1319 original palliative medicine research articles published by U.S. authors from 2011 to 2013, representing 2120 palliative care researchers. Of these, 832 articles (63%) were published in palliative medicine journals and 487 (37%) in general medicine and relevant subspecialty journals (Fig. 1). Thirty-nine percent of the authors identified had NIH grants identified in the RePORTER search.
FIG. 1.
Comparison of published palliative care articles, 2006–2010 versus 2011–2015.
Types of grants
Of the awards identified, 60 (13.1%) were career development awards (45 awarded to early stage investigators and 15 to experienced investigators), 40 (8.7%) were training grants, 66 (14.3%) were pilot grants, 139 (30.2%) were research project grants, 27 (5.9%) were education project grants, and 68 (14.8%) were Center grants (Table 1). Across all institutes, 2.5% of all K23 grants, 0.5% of all R01 grants, and 0.4% of all R21 grants supported palliative care research. In Table 1, those cells with the number zero represent that there were no grants funded in those categories, which could represent no grant funded and/or the mechanism was not funded by that Institute during the study period. There are no data to track the period for which each institute funded each mechanism.
Table 1.
Selected NIH-Funded Awards for Palliative Care Research by Institute from 2011 to 2015
| Institute | All grants | Early-stage CDAa | Experienced investigator CDAb | Training grantsc | Pilot grantsd | Research project grantse | Education project grantsf | Center grantsg |
|---|---|---|---|---|---|---|---|---|
| All institutes | 461 | 45 (9.8) | 15 (3.3) | 40 (8.7) | 66 (14.3) | 139 (30.2) | 27 (5.9) | 68 (14.8) |
| NIA | 115 (24.9) | 28 (62.2) | 7 (46.7) | 3 (2.6) | 25 (37.9) | 20 (14.4) | 0h | 21 (18.3) |
| NCI | 143 (31.0) | 7 (15.7) | 6 (40.0) | 5 (3.5) | 18 (27.3) | 33 (23.7) | 26 (96.3) | 36 (25.2) |
| NHLBI | 17 (3.7) | 2 (4.4) | 1 (6.7) | 4 (23.5) | 1 (1.5) | 8 (5.8) | 0h | 0h |
| NIMH | 5 (1.1) | 1 (2.2) | 0h | 0h | 1 (1.5) | 2 (1.4) | 0h | 0h |
| NINR | 112 (24.3) | 2 (4.4) | 1 (6.7) | 26 (23.2) | 12 (18.2) | 58 (41.7) | 0h | 8 (7.1) |
| NIDDK | 6 (1.3) | 1 (2.2) | 0h | 1 (16.7) | 1 (1.5) | 1 (0.7) | 0h | 2 (33.3) |
| Other | 63 (13.7) | 0h | 0h | 1 (1.6) | 2 (3.0) | 7 (5.0) | 0h | 1 (1.6) |
Early-stage investigator CDA: K01, K07, K08, and K23.
Experienced investigator CDA: K05 and K24.
Training grants: F31, F32 and T32.
Pilot grants: R21 and R03.
Research project grants: R01.
Education project grants: R25.
Center grants: P01, P30, P50, U01, U10, U19, U24, U2C, U54, UG1, and UH3.
The number zero reflects that there were no grants funded in those categories, which may be because these Institutes did not fund the mechanism or did not fund the mechanism during the study period. This information could not be tracked by year of the study period.
CDA, career development award; NCI, National Cancer Institute; NHLBI, National Heart Lung Blood Institute; NIA, National Institute on Aging; NIDDK, National Institute on Diabetes, Digestive and Kidney Diseases; NIMH, National Institute of Mental Health; NINR, National Institute for Nursing Research.
Funding by research topic area
Of 461 identified NIH grants, 126 (27.3%) funded health services research evaluating models of care; 78 (16.9%) funded decision-making and communication studies; 58 (12.6%) addressed pain, other symptoms, and quality of life; 42 (9.1%) supported education and training in palliative medicine; 30 (6.5%) funded studies related to caregivers and families of seriously ill patients; 27 (5.9%) funded pediatrics studies; 25 (5.4%) funded studies examining psychological, spiritual, and emotional symptoms; 13 (2.8%) funded studies related to measurements or instrument development; and 53 (11.5%) funded other areas of research.
Funding by institute
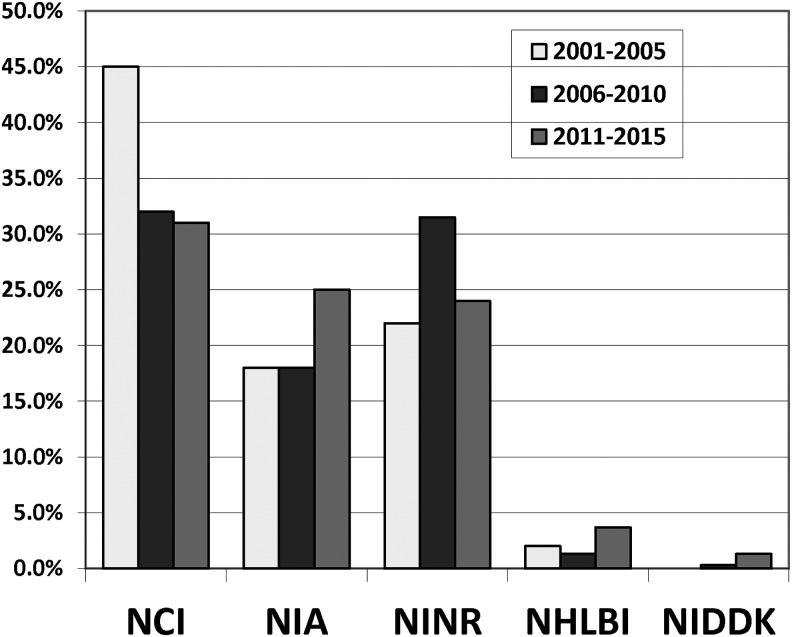
Three NIH institutes (National Cancer Institute [NCI], NINR, and National Institute on Aging [NIA]) accounted for 80.2% of all awards distributed. One hundred forty-three (31.0%) were funded by NCI (0.5% of all NCI research grants awarded); 112 (24.3%) by NINR (8.1% of all NINR grants); 115 (24.9%) by NIA (1.3% of all NIA grants); and 63 (13.7%) by other Institutes/Centers. Specifically, 17 (3.7%) grants on palliative medicine research were funded by National Heart Lung Blood Institute (NHLBI), which represent 0.08% of all NHBLI grants, and 6 (1.3%) grants were funded by National Institute on Diabetes, Digestive and Kidney Diseases (NIDDK), which represent 0.04% of all NIDDK grants (Fig. 2).
FIG. 2.
Comparison of funding by institute, 2001–2005 versus 2006–2010 versus 2011–2015. NCI, National Cancer Institute; NHLBI, National Heart Lung Blood Institute; NIDDK, National Institute on Diabetes, Digestive and Kidney Diseases; NIA, National Institute on Aging; NINR, National Institute for Nursing Research.
Discussion
This study serves to update our prior research examining NIH funding in palliative medicine. From 2011 to 2015, we identified 461 unique grants—an overall increase of 70 funded grants (17.9%) compared to 2006–2010 and 43 (10%) from 2001 to 2005. The numbers of funded investigators also increased from 109 in our first study to 294 in our second, to 334 investigators in this report—an increase of 185 (170%) from our first study and 40 (14%) from our second. Most encouragingly, given the need to expand the palliative care research workforce, compared to 2006–2010, the number of early stage investigators receiving career development awards nearly doubled (24–45 grants) (Table 1). In addition, there has been an investment in palliative medicine through Center grants, including those in the P series, which support large, multiproject efforts, and those in the U series, which support research cooperative networks. It is important to note that the strides made by palliative medicine occurred during a time when NIH paylines were consistently declining and indeed, the period 2011–2015 corresponded to the lowest rates of funding in NIH history.
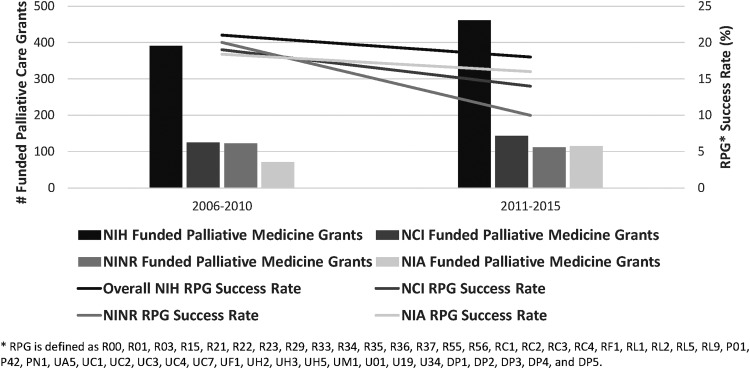
The fact that funding for palliative care research increased across almost all institutes is notable given the funding climate at NIH during this time period. Because NCI, NINR, and NIA continue to fund the majority of palliative medicine research (80% of all grants) grants, we compared the mean success rates for new research program grants by institute from 2006–2010 to 2011–2015, to better understand the funding climate (https://report.nih.gov/success_rates/Success_ByIC.cfm). Success rates are defined as the percentage of reviewed grant applications that receive funding (https://report.nih.gov/uploadDocs/NIH Success Rate Definition 2012.pdf)
The percentage of palliative care grants funded by NCI and NINR remained relatively stable from our second report to this one, although the mean success rate of grants awarded by NCI and NINR decreased considerably between the two time periods (NCI: 17.3% [2006–2010] to 12.6% [2011–2015]; NINR: 18.6% [2006–2010] to 9.9% [2011–2015]). Palliative medicine funding by NIA increased from 71 grants (18.2%) to 115 grants (24.9% of all palliative medicine-funded grants); this increase occurred in the context of the NIA's average payline's more modest decrease (16.3% [2006–2010] to 13.9% [2011–2015]) (Fig. 3).
FIG. 3.
Comparison of the number of NIH-funded palliative medicine grants by institute, with mean NIH RPG success rate by institute, 2006–2010 versus 2011–2015. RPG, new research program grants.
Although only 20% of all palliative medicine grants were funded by other institutes, there were considerable increases in the number of grants by these institutes. For example, palliative medicine funding by NHLBI increased from 5 grants (1.3%) to 17 grants (3.7% of all palliative medicine-funded grants) and by NIDDK from 1 grant (0.3%) to 6 grants (1.3% of all palliative medicine-funded grants) (Fig. 1). In light of this funding climate, increased funding for palliative care research is notable and draws attention to the increasing strength and rigor of submitted applications.
Our results also highlight the growth of the palliative care scientific workforce. The number of published palliative care researchers not only increased by over 1.5-fold from 2006–2010 to 2011–2015 (839–2120, respectively), but the percentage of authors who received NIH funding support increased by over 85% (1% in 2006–2010 to 39% in 2011–2015). Furthermore, these authors reached a more extended audience with their research. Whereas the overall number of published articles stayed relatively the same, the percentage of articles published in nonpalliative care specialty journals nearly tripled from 13% in 2006–2010 to 37% in 2011–2015.
Finally, compared to our earlier reports, this study found a broadening in the areas of research from a nearly exclusive focus on pain and symptom management (29.3% [2001–2005], 19.7% [2006–2010], and 12.6% [2011–2015]) to new areas of growth in pediatrics (1.9% [2001–2005], 6.4% [2006–2010], and 5.9% [2011–2015]), decision making and communication (10.1% [2001–2005], 13.3% [2006–2010] and 16.9% [2011–2015]), and health services research (8.6% [2001–2005], 18.2% [2006–2010], and 27.3% [2011–2015]). These results reflect important attention to all domains of palliative care identified by the National Consensus Project17 by palliative care investigators.
What factors might have created these changes?
The reasons behind the observed growth in palliative care research are likely multifactorial and it is difficult to assign credit to specific initiatives. Clearly, the growth and development of the entire field, the origins of nonhospice palliative medicine within academic medical centers, and the strengthening of these academic departments have played a fundamental role in enhancing the evidence base. The establishment and growth of the NINR-funded Palliative Care Research Cooperative at the University of Colorado Denver, Duke University, and the University of California, San Francisco, have provided an unprecedented platform to support multisite palliative care trials and provide the training ground for early stage clinical trialists interested in palliative care research. Indeed, over the past seven years, the Cooperative has supported the submission of 51 NIH research applications and 27 studies have been conducted under the Cooperative's infrastructure.10
Private sector philanthropy, like it did for the development of palliative care clinical and educational programs, has also played a critical role in stimulating palliative care research. The NPCRC in New York, established by the Joseph S. and Emily Davie Kornfeld Foundation and subsequently supported by investments from over 15 individual philanthropists and foundations, has served both as a funder and organizing home for palliative care research. Since 2007, NPCRC has funded 83 investigators from 42 institutions and 20 states at a cost of $13.7 million. These 83 investigators have subsequently secured 124 federal grants totaling $157 million in direct costs. Similarly, the American Cancer Society, the single largest funder of research outside the federal government, continues to invest over $500 thousand annually through its various research funding mechanisms to support the science of palliative care.
Finally, strong leadership within NIH, and particularly NINR and NIA, has led important initiatives, including several workshops outlining priorities for palliative care research,8 Program Announcements focused specifically on palliative care (an R01 [https://grants.nih.gov/grants/guide/pa-files/PA-17-225.html], and an R21 [https://grants.nih.gov/grants/guide/pa-files/PA-17-226.html]). These funding opportunities reflect a commitment by these institutes to fund research that focuses on the development new tools, methods, and models for palliative care in geriatric populations, and the aforementioned Palliative Care Research Cooperative.10 The Patient Centered Outcomes Research Institute's recent funding announcements in palliative care are also major steps forward toward enhancing the evidence base for the seriously ill. (www.pcori.org)
Limitations
This study has several limitations that should be noted. First, our study was designed specifically to identify palliative medicine studies and researchers and thus did not include studies in related areas that might have implications for palliative medicine research (e.g., development of palliative chemotherapy drugs and treatments focused on surgical pain). These criteria potentially excluded relevant research in related areas. Second, we focused specifically on studies in adult internal medicine such that we may have underreported funding in pediatrics, psychiatry, surgery, neurology, and anesthesiology. Third, because we identified researchers from published articles, it is possible that we did not identify funding for junior investigators who have not yet published their results. We suspect, however, that the number of such researchers is small. Fourth, there were some changes to data mining used in the NIH RePORTER search engine over time, which would affect our ability to trend changes in NIH funding to palliative care over time. Furthermore, the key words selected (palliative care, end-of-life-care, hospice, and end of life) are not part of the Research-, Condition-, and Disease Categorization System-defined research/disease categories. It is therefore possible that exclusive use of the key words may miss some NIH awards in palliative medicine. Finally, we did not include newer funding sources, including PCORI and CMMI.
Conclusions
In conclusion, our third report of NIH funding of palliative medicine research shows continued improvement with respect to the numbers of NIH-funded palliative care investigators (particularly early stage investigators); the proportion of palliative medicine research funded by NIH; and the interest in palliative medicine research by nonpalliative care specialty journals, suggesting broader dissemination of the palliative care evidence base. Despite these positive findings, the overall number of NIH grants awarded for palliative medicine research remains low, and in turn, federal funding for palliative medicine research is still inadequate. Again, fewer than 1% of all grants awarded overall by the NIH and the NCI, NHLBI, the NIDDK, and the National Institute of Neurological Disorders and Stroke (four of the largest Institutes and those representing six of the eight leading causes of death in the United States, excluding accidents)18 were awarded to investigators performing palliative medicine research. These percentages have remained unchanged for over 15 years. Further efforts are needed to develop the evidence base for palliative medicine, including appropriate representation of palliative care researchers on relevant NIH study sections, dedicated funding for palliative research across major NIH institutes, and ongoing and increased investments by private foundations to support pilot research and the career development of early stage investigators.
Acknowledgments
Ms. E.B. was supported by the Medical Student Training in Aging Research (MSTAR) Fellowship administered by American Federation for Aging (AFAR). Dr. L.P.G. received support from the National Institute on Aging (NIA) (K23AG049930). Dr. R.S.M. received support from the Mount Sinai Older Adults Independence Center (P30AG028741), the National Institute on Aging (NIA) (R24AG044300), and the American Cancer Society Clinical Research Professor Grant.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Morrison R: State-By-State Report Card on Access to Palliative Care in Our Nation's Hospitals. New York: Center to Advance Palliative Care, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abernethy AP, Aziz NM, Basch E, et al. : A strategy to advance the evidence base in palliative medicine: Formation of a palliative care research cooperative group. J Palliat Med 2010;13:1407–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavalieratos D, Corbelli J, Zhang D, et al. : Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA 2016;316:2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NINR (National Institute of Nursing Research). NNIoN. Building momentum: The science of end-of-life and palliative care. A review of research trends and funding, 1997–2010. 2013. www.ninr.nih.gov/sites/www.ninr.nih.gov/files/NINR-Building-Momentum-508.pdf (last accessed August1, 2017)
- 5.Gelfman LP, Morrison RS: Research funding for palliative medicine. J Palliat Med 2008;11:36–43 [DOI] [PubMed] [Google Scholar]
- 6.Gelfman LP, Du Q, Morrison RS: An update: NIH research funding for palliative medicine 2006 to 2010. J Palliat Med 2013;16:125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison RS, Meier DE: The National Palliative Care Research Center and the Center to Advance Palliative Care: A partnership to improve care for persons with serious illness and their families. J Pediatr Hematol Oncol 2011;33 Suppl 2:S126–S131 [DOI] [PubMed] [Google Scholar]
- 8.Morrison RS, Zieman S: Introduction to series. J Palliat Med 2017;20:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison RS: Research priorities in geriatric palliative care: An introduction to a new series. J Palliat Med 2013;16:726–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie CL, Pollak KI, Kehl KA, et al. : Better together: The making and maturation of the Palliative Care Research Cooperative Group. J Palliat Med 2017;20:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson LC, Bull J, Wessell K, et al. : Strategies to support recruitment of patients with life-limiting illness for research: The Palliative Care Research Cooperative Group. J Pain Symptom Manage 2014;48:1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeBlanc TW, Kutner JS, Ko D, et al. : Developing the evidence base for palliative care: Formation of the Palliative Care Research Cooperative and its first trial. Hosp Pract (1995) 2010;38:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan AM, Gadmer NM, Block SD: The project on death in America faculty scholars program: A report on scholars' progress. J Palliat Med 2009;12:155–159 [DOI] [PubMed] [Google Scholar]
- 14.Aulino F, Foley K: The project on death in America. J R Soc Med 2001;94:492–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Research Porfolio Online Reporting Tools (RePORT). http://projectreporter.nih.gov/reporter.cfm (Last accessed July11, 2012)
- 16.National Consensus Project for Quality Palliative Care: Clinical Practice Guidelines for Quality Palliative Care. New York, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Forum NQ: National Consensus Project for Quality Palliative Care: Clinical Practice Guidelines for quality palliative care, executive summary. J Palliat Med 2004;7:611–627 [DOI] [PubMed] [Google Scholar]
- 18.Leading Causes of Death. 2017. www.cdc.gov/nchs/fastats/leading-causes-of-death.htm (Last accessed June8, 2017)