Abstract
Purpose
Consumption of a high-fat, high-sugar diet and sedentary lifestyle are correlated with bulk arterial stiffening. While measurements of bulk arterial stiffening are used to assess cardiovascular health clinically, they cannot account for changes to the tissue occurring on the cellular scale. The compliance of the subendothelial matrix in the intima mediates vascular permeability, an initiating step in atherosclerosis. High-fat, high-sugar diet consumption and a sedentary lifestyle both cause micro-scale subendothelial matrix stiffening, but the impact of these factors in concert remains unknown.
Methods
In this study, mice on a high-fat, high-sugar diet were treated with aerobic exercise or returned to a normal diet. We measured bulk arterial stiffness through pulse wave velocity and subendothelial matrix stiffness ex vivo through atomic force microscopy.
Results
Our data indicate that while diet reversal mitigates high-fat, high-sugar diet-induced macro- and micro-scale stiffening, exercise only significantly decreases micro-scale stiffness and not macro-scale stiffness, during the time-scale studied.
Conclusions
These data underscore the need for both healthy diet and exercise to maintain vascular health. These data also indicate that exercise may serve as a key lifestyle modification to partially reverse the deleterious impacts of high-fat, high-sugar diet consumption, even while macro-scale stiffness indicators do not change.
Keywords: Subendothelial matrix, arterial stiffness, pulse wave velocity, atomic force microscopy, High-fat, high-sugar diet, atherosclerosis
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, and atherosclerosis, the development of lipid-rich plaques in large arteries, is a primary contributor to CVD [1]. Arterial stiffening is an independent predictor of CVD onset, and is measured clinically by the gold standard pulse wave velocity (PWV), a measurement of the velocity of a pressure wave as it travels down the aorta along the arterial tree [2]. CVD risk factors, such as consumption of a Western diet, advanced age, and lack of exercise, promote macro-scale arterial stiffening [3–5]. We were the first to show that mice that consume a high-fat, high-sugar (HFHS) diet exhibit an increase in macro-scale arterial stiffness, as measured in vivo by PWV, as well as stiffening of the subendothelial matrix, as measured on aortic rings at the micro-scale level by atomic force microscopy [6]. We also showed that reversal to a normal diet can return the vessel to its original stiffness [6]. In addition, after consumption of a Western diet, implementation of an exercise regimen has been shown to decrease [7] or to cause no effect [8] on macro-scale arterial stiffness, however less is known about the effects of exercise on the specific mechanics of the arterial intima [5].
Endothelial cells respond to the micro-scale extracellular mechanical cues of the subendothelial matrix on which they lay. Subendothelial matrix stiffening due to aging causes a loss of endothelial cell-cell junctional integrity, increased vascular permeability, and leukocyte transmigration [9–11]; subsequent cholesterol accumulation in the arterial wall is an initiating step in atherosclerotic plaque progression [12]. Therefore, understanding the complex effects of cardiovascular risk factors on micro-scale intimal mechanics is of clinical relevance. Given the rise of CVD and obesity in recent decades [13], there is a need to understand the impact of lifestyle factors on arterial function. We have previously demonstrated that age-induced subendothelial matrix stiffening can be mitigated by an exercise regimen [14], and that subendothelial matrix stiffness is increased by consumption of a HFHS diet [6]. Here, we hypothesize that an exercise regimen can mitigate diet-induced subendothelial matrix stiffening. Since the subendothelial matrix is a thin layer in the intima layer, we postulate that stiffness changes in this layer would not affect whole vessel mechanics. On the other hand, macro-scale stiffness measures, such as PWV, may correlate with changes in the subendothelial matrix stiffness in certain conditions, as in our previous studies [6, 14]. Therefore, to compare the changes in the subendothelial matrix with known clinical arterial stiffness metrics, we also measure PWV and blood pressure in normal diet- and HFHS-fed animals.
Methods
Animal models
All animal work has been approved by Cornell University Institutional Animal Care and Use Committee (IACUC). For diet and exercise groups, C57Bl/6 male mice (Jackson Labs, #00664) were obtained at 8 weeks old and ear-notched for identification. Mice had access to food and water ad libitum and maintained in a 12-hour light/dark cycle. In the diet-period, mice were fed a HFHS diet with 35.5% fat and 18.2% sucrose, or a ND with 4.5% fat and 0% sucrose (D09071702 and D09071703 from Research Diets, respectively) for 9 weeks. The fats used in both diets are composed of 33.95% saturated fatty acids, 37.20% monounsaturated fatty acids, and 28.85% polyunsaturated fatty acids. We have previously used these animal and diet conditions, and did not observe plaque development in the arteries [6]. In the exercise-period, mice maintained their initial diet (ND or HFHS Sed), changed diets (HFHS/ND), or underwent an exercise regimen (HFHS Ex) for 8 weeks. Exercise and sedentary mice were house together while different diet group mice were housed in separate cages. Animals were weighed approximately three times each week; their weights were averaged and normalized to their pre-diet baseline weight. Eighteen months old C57Bl/6 mice used for the atomic force microscopy loading rate data collection were acquired from the National Institute on Aging, were kept on standard chow (Harlan Teklad 7912) and did not go through any exercise regimen before tissue analysis.
Exercise regimen
A swimming exercise regimen was employed as previously described [14]. Briefly, mice swam in groups of 2 – 5 over an 8-week period. Mice were first acclimated to the environment with three 3-minute swim sessions. The swimming regimen consisted of swimming 5 days per week for 10 minutes per day in week 1, 30 minutes per day in weeks 2–3, and 45 minutes per day in weeks 4–8. Sedentary control mice were kept in their cages while their exercised cage-mates were swimming in the same room. Mice did not swim on days of PWV/blood pressure measurements.
Blood pressure measurements
A non-invasive blood pressure tail cuff system (Kent Scientific) was used to measure blood pressure on non-sedated mice based on manufacturer’s guidelines. Briefly, mice were first acclimated to the constraint tubes in days prior to blood pressure measurements. During the procedure, 10 tail cuff inflation-deflation cycles with 20-second cuff deflation time for each animal were performed. The software (Kent Scientific CODA) was used to screen recorded pressure tracings. Cycles with a blood tail volume below 10 μL were rejected.
Pulse wave velocity (PWV) measurements
Macro-scale arterial stiffness was measured in vivo with Vevo 2100 Doppler ultrasound, as we previously described [6]. Briefly, mice were kept lightly anaesthetized with 0.5 – 2 % isoflurane and supine on a heating platform. Hair in the abdominal region was removed with a shaving cream (Nair). The renal vein was used as an anatomical reference to select a longitudinal segment of abdominal aorta <10 mm long. Flow waves at one proximal and one distal location along the aorta, and simultaneous ECG, were acquired with a MS550D transducer for at least 5 cardiac cycles for each mouse. VevoLab v1.7.1 software was used to measure the distance between the two points and the arrival times between the peak of the R wave and the foot of each flow wave. PWV was calculated as the distance divided by the difference of the two arrival times (transit time). To note, heart rates were kept at comparable levels (400–500 bpm) in all mice during flow wave acquisitions.
Subendothelial matrix mechanics
The subendothelial matrix elastic modulus was measured using atomic force microscopy (AFM) (Asylum MFP-3D) on the subendothelial matrix of freshly isolated thoracic aortas, as we previously described [14]. Briefly, after perfusion with ice-cold phosphate buffered saline (PBS), the thoracic aorta was removed and cleaned from fat. The aorta was cut longitudinally, glued onto a Petri dish (Loctite Super Glue), and covered in PBS at room temperature. The endothelial cells were gently scraped off the luminal side to expose the underlying subendothelial matrix, as previously described [15]. A cantilever (Novascan) with a 10 μm diameter polystyrene bead was used for indentations. The cantilevers were supplied with an approximate spring constant of 0.12 N/m, and were calibrated before the experiments at 0.11 ± 0.05 N/m. Indentations of the tissue were made at a velocity of 1 μm/sec and a loading rate of approximately 40 nN/sec. Data were fit to the Hertz Model using Asylum software and assuming a Poisson’s ratio of 0.5 to determine the elastic modulus, as previously described [9, 14, 15].
Loading rate dependency of the subendothelial matrix elastic modulus
For the loading rate data collection, experiments were conducted separately from those on the diet mice cohorts. Thoracic aortas from aged mice (n=3) were used to collect the loading rate dependency data. A range of AFM loading rates from ~5 to 76 nN/s was used to determine the force rate dependence on the elastic modulus of the tissue.
Statistical Analysis
Data were analyzed and graphed on GraphPad Prism 7 software and tested for normality using a Shapiro-Wilk test. For two group comparisons of normally distributed data, a Student’s t-test was used. For multiple group comparisons, one-way ANOVA with Dunnett’s multiple comparisons test comparing to the normal diet (ND) group was used. P-values < 0.05 were considered significant. Linear correlations were found to be significant with a Pearson Correlation p-value < 0.05 for all data groups combined in the figure. The linear fit R2 value was measured for the analysis of multiple studies for loading-rate dependence.
Results
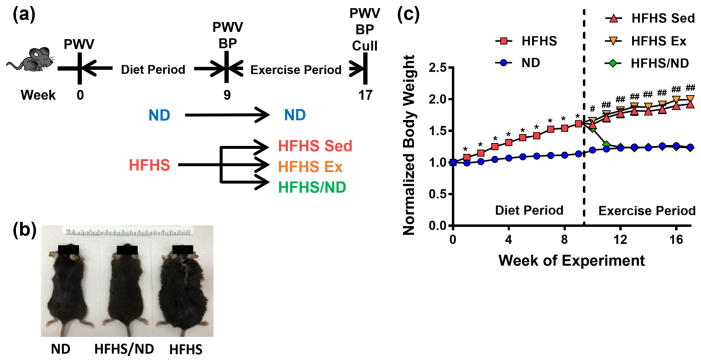
Mouse body weight increases with high-fat, high-sugar diet consumption and is mitigated by diet reversal
The rates of CVD and obesity are increasing in society [13], and risk factors such as poor diet and sedentary lifestyle can cause increased body weight and macro-scale arterial stiffening [7, 8]. Here, mice were fed a normal (ND) or a high-fat, high-sugar (HFHS) diet during a nine-week period, then separated into four cohorts for the following 8-week exercise period. Specifically, mice either remained sedentary while on their original diet (ND or HFHS Sed), returned to a ND (HFHS/ND), or exercised while on a HFHS diet (HFHS Ex) (Figure 1a). Body weights increased in mice fed the HFHS diet for the entire study (Figure 1b). Body weight, normalized to each animal’s initial starting weight, increased significantly after two weeks on the HFHS diet, and returned to baseline within two weeks of change from HFHS to ND consumption (Figure 1c). The body weight of mice that remained sedentary on a HFHS diet throughout the study remained elevated, even following the exercise regimen (final normalized body weight mean ± SEM: ND 1.24 ± 0.05, n = 4; HFHS/ND 1.23 ± 0.06, n = 6; HFHS Sed 1.92 ± 0.14, n = 7; HFHS Ex 2.00 ± 0.06, n = 7).
Fig. 1.
Mouse body weight increases after high-fat, high-sugar (HFHS) diet consumption. (a) During the diet period, mice consumed a normal diet (ND) or HFHS diet for two months. During the exercise period, the mice maintained their initial diet and remained sedentary (ND and HFHS Sed), underwent an 8-week exercise regimen (HFHS Ex) or switched diets while remaining sedentary (HFHS/ND). (b) Increased body weight due to consumption of a HFHS diet can be easily visualized. (c) Mouse body weight normalized to each mouse’s starting weight was significantly increased with HFHS diet and decreased after return to ND, * p < 0.05 (Student’s t-test), # p < 0.05 (ANOVA, all groups are significantly different from ND), ## p < 0.05 (ANOVA, HFHS Sed and HFHS Ex groups are significantly different from ND), n = 4 to 7 mice per diet/exercise group
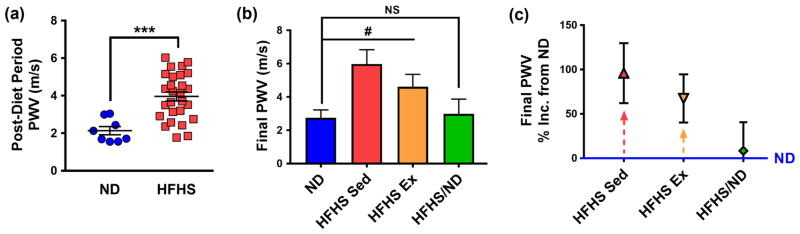
High-fat, high-sugar diet-induced arterial stiffening is mitigated by diet reversal, but not by exercise
To investigate the effects of diet reversal and exercise on arterial stiffening following a high-fat, high-sugar diet, non-invasive PWV measurements were collected after 9 weeks on diet and at the conclusion of the study. Following the first two months of diet, PWV increased in mice on a HFHS diet compared to their ND counterparts (mean ± SEM: ND 2.14 ± 0.22 m/s, n = 8 and HFHS 3.96 ± 0.24 m/s, n = 27) (Figure 2a). At the conclusion of the study, the HFHS diet-induced increase in PWV was mitigated by diet reversal, while PWV remained elevated for the HFHS-fed mice regardless of exercise (mean ± SEM: ND 2.75 ± 0.47 m/s, n = 4; HFHS/ND 2.98 ± 0.89 m/s, n = 5; HFHS Sed 6.00 ± 0.86 m/s, n = 6; HFHS Ex 4.61 ± 0.75 m/s, n = 7) (Figure 2b). These data indicate that this 8-week aerobic exercise regimen was not sufficient to fully reverse the deleterious impact of HFHS diet consumption on macro-scale stiffening. PWV in the HFHS diet mice after the exercise regimen remained elevated by 67.4 ± 27 % (mean ± SEM, n = 7) compared to the ND cohort, whereas the HFHS sedentary mice experienced a 95.9 ± 34 % (n = 7) increase compared to ND values (Figure 2c). Interestingly, PWV in the diet reversal group returned to baseline by the conclusion of the study (within 8.34 ± 32 % of ND values, n = 5), in accordance with our previous findings that reversing to a healthy diet may be a beneficial treatment for diet-induced macro-scale stiffening [6].
Fig. 2.
Pulse wave velocity (PWV) is affected by diet consumption, but not by the exercise regimen. (a) By the end of the diet period, PWV increased in mice on a HFHS diet compared to those on a ND, *** p < 0.0005 (Student’s t-test), n = 8 mice for ND and 27 mice for HFHS group. (b) After 8 weeks in the exercise period, PWV decreased in mice that switched from a HFHS diet to ND, while PWV remained elevated for HFHS diet mice, with or without the applied exercise regimen, # p < 0.05 (ANOVA, HFHS Sed and HFHS Ex groups are significantly different from ND), NS = not significantly different. (c) Mice remaining on a HFHS diet with or without the exercise regimen maintained an elevated PWV at the end of the study, as indicated by the high percentage increase of these groups compared to the ND cohort. All error bars in the figure are SEM and n = 4 to 7 mice per diet/exercise group
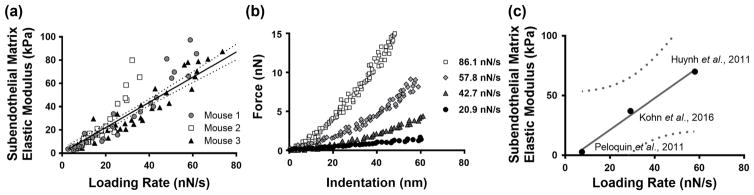
Subendothelial matrix stiffness is loading rate-dependent
Since increased substrate stiffness has been demonstrated to alter endothelial barrier function [9,10], we sought to analyze the mechanical properties of subendothelial matrix of the mouse aorta using atomic force microscopy (AFM). Using aortas of 18-month old mice, we found that the elastic modulus of the subendothelial matrix is loading rate-dependent, and a positive linear correlation exists between the measured elastic modulus and the loading rate (Figure 3a). Representative force versus indentation curves demonstrate an increased slope at higher loading rates (Figure 3b). To confirm this holds true across other artery samples, measurements from several published studies were plotted, and a linear correlation between measured subendothelial matrix elastic modulus and loading rate yielded an R2 value of 0.9917 (Figure 3c) [9, 14, 15]. These data indicate that the loading rate during AFM indentation should be taken into account when assessing the mechanics of the subendothelial matrix.
Fig. 3.
The elastic modulus of the murine aorta subendothelial matrix is loading rate dependent. (a) The measured elastic modulus of the subendothelial matrix increases with higher loading rates, as shown in three different mice; points collected from 10 locations on each aorta; significant linear trend shown with 95% confidence interval bands, p < 0.0001 (Pearson Correlation). (b) At a representative location on a mouse aorta, atomic force microscopy force versus indentation curves demonstrate a steeper slope with higher loading rates. (c) Independent studies of subendothelial matrix stiffness of the aortic intima demonstrate its loading rate dependence. Aged murine aorta data were used for Kohn et al., 2016 and Huynh et al., 2011. Mature bovine aorta data were used for Peloquin et al., 2011. The linear trend with 95% confidence interval bands are presented, and the linear fit R2 value equals 0.9917.
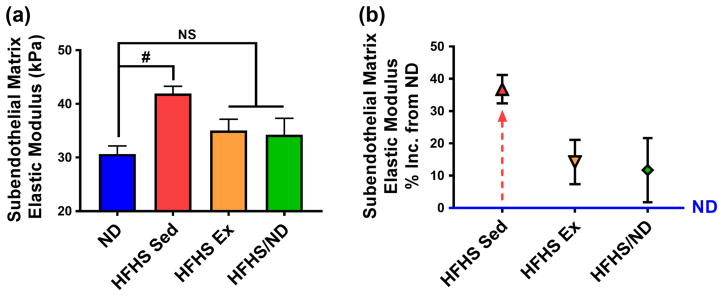
High-fat, high-sugar diet-induced subendothelial matrix stiffening is mitigated by diet reversal and exercise
To investigate whether diet reversal and exercise can reverse the HFHS-induced micro-scale stiffening, we probed the subendothelial matrix of diet and exercise cohorts described above (Figure 1a). We used a loading rate of ~40 nN/sec, which provides an average maximum force of 4 nN on the tissue, based on methods from a previous study [14]. HFHS diet-induced subendothelial matrix stiffening was mitigated by diet reversal or, to a similar extent, by the aerobic exercise regimen (mean ± SEM: ND 30.7 ± 1.5 kPa, n = 3; HFHS/ND 34.3 ± 3.0 kPa, n = 4; HFHS Sed 41.9 ± 1.3 kPa, n = 6; HFHS Ex 35.0 ± 2.1 kPa, n = 7) (Figure 4a). Importantly, while macro-scale stiffness returned to baseline only after diet reversal, the subendothelial matrix stiffness returned to baseline after diet reversal or the exercise regimen. In fact, the subendothelial matrix stiffness remained elevated only 14.2 ± 6.7 % (mean ± SEM, n = 7) in HFHS mice after the exercise regimen, and 11.7 ± 9.9 % (n = 4) in the diet reversal group, compared to the ND cohort; whereas, the HFHS sedentary group experienced a 36.8 ± 4.4 % (n = 6) increase (Figure 4b). Taken together, these data indicate that exercise while remaining on a HFHS diet has a significant effect on subendothelial matrix de-stiffening, but does not affect whole arterial wall de-stiffening to a similar extent.
Fig. 4.
Increased subendothelial matrix elastic modulus due to HFHS diet consumption is rescued by the exercise regimen or a return to ND. (a) Micro-scale subendothelial matrix elastic modulus measured with atomic force microscopy increased after HFHS diet consumption, and was recovered with diet reversal or an exercise regimen, # p < 0.05 (ANOVA, HFHS Sed group is significantly different from ND), NS = not significantly different. (b) Mice remaining on a HFHS diet without the exercise regimen maintained an elevated subendothelial matrix stiffness at the end of the study, as indicated by the high percentage increase of this group compared to the ND cohort. All error bars in the figure are SEM and n = 4 to 7 mice per diet/exercise group
Diastolic blood pressure and mean arterial pressure are elevated after two months of HFHS diet consumption
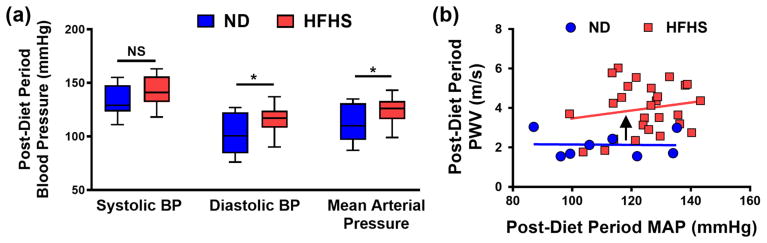
Previous studies have emphasized the importance of monitoring blood pressure when measuring bulk stiffness metrics, such as PWV [16–20]. Therefore, we monitored the effects of diet and exercise on blood pressure in resting, conscious mice with a non-invasive tail cuff system. After two months of HFHS diet consumption, diastolic blood pressure (DBP) (mean ± SEM: ND 102 ± 6.8 mmHg, n= 8; HFHS 117 ± 2.2 mmHg, n = 27) and mean arterial pressure (MAP) (ND 112 ± 6.3 mmHg, n= 8; HFHS 125 ± 2.1 mmHg, n = 27) increased compared to the ND cohort, although there was no detectable change in systolic blood pressure (SBP) (ND 133 ± 5.3 mmHg, n= 8; HFHS 142 ± 2.4 mmHg, n = 27) (Figure 5a). Interestingly, at this time point PWV was elevated in HFHS-fed mice at a given MAP value compared to the ND cohort, as demonstrated by the upward shift in the stiffness versus blood pressure linear trends (Figure 5b). These data indicate that two months of HFHS diet consumption was sufficient to induce increases in MAP in mice, but PWV remain largely independent of MAP.
Fig. 5.
After two months of consuming the HFHS diet, elevated blood pressure is detected. (a) Diastolic blood pressure and mean arterial pressure (MAP) increased after two months on a HFHS diet compared to the ND cohort, but systolic blood pressure did not significantly increase; box and whisker plots shown with min/max error bars, * p < 0.05 (Student’s t-test); NS = not significantly different. (b) Following the diet period, a plot of PWV versus MAP values for each mouse indicates an upward shift in the PWV of HFHS diet mice at a given MAP compared to the ND cohort; each data point represents the average value of one mouse, and the black arrow indicates an upward shift between the linear regressions. n = 8 mice for ND and 27 mice for HFHS group
Exercise did not mitigate HFHS diet-induced elevated blood pressure at the conclusion of the study
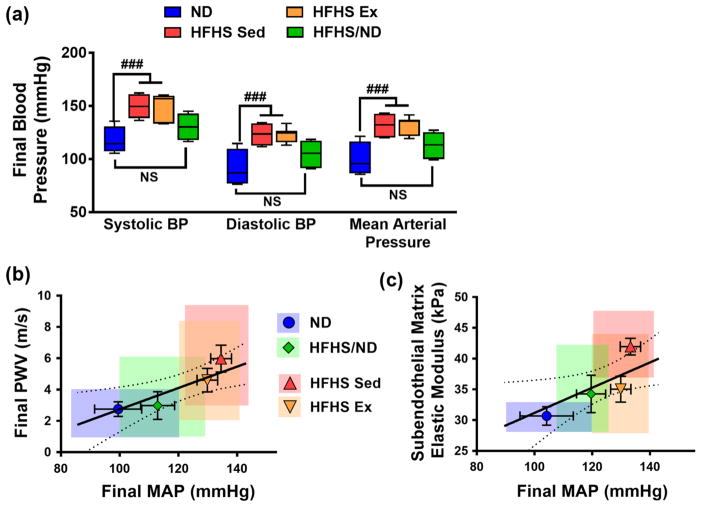
Interestingly, SPB, DBP and MAP were higher in mice that remained on the HFHS diet for the duration of the study, regardless of exercise (Figure 6a). Taken together, our results demonstrate that while return to ND consumption was effective at reducing blood pressure and body weight within the time course of the study, the exercise regimen did not reduce either of these metrics significantly. We further compared the effects of blood pressure (specifically MAP) with the macro- and micro-scale stiffness measurements of the diet and exercise groups. At the conclusion of the study, both PWV and subendothelial matrix elastic modulus were linearly correlated with MAP (Figures 6b and 6c). It is also important to note that SBP was not significantly elevated after the first two months on the HFHS diet, but was elevated by the conclusion of the study (mean ± SEM: ND 117 ± 6.4 mmHg, n= 4; HFHS Sed 150 ± 4.1 mmHg, n = 7). PWV increased after 9 weeks on the HFHS diet, while SBP did not significantly increase until the 17-week time point, in accordance with our previous findings where systolic hypertension followed elevated PWV [6]. While the link between arterial stiffness and the development of hypertension is complex [17], our data suggest that initial increases in arterial stiffness may play a role in the subsequent progression of systolic hypertension in HFHS-fed mice.
Fig. 6.
At the conclusion of the study, elevated blood pressure is detected in both HFHS diet groups, regardless of the application of the exercise regimen. (a) Systolic and diastolic blood pressure (BP), as well as mean arterial pressure (MAP), increased in the HFHS Sed and HFHS Ex groups by the conclusion of the study, ### p < 0.005 (ANOVA, HFHS Sed and HFHS Ex groups are significantly different from ND); NS = not significantly different, error bars are SEM. (b) At the conclusion of the study, MAP is linearly correlated with PWV (Pearson Correlation: p = 0.0112, r = 0.5298) and (c) subendothelial matrix elastic modulus (Pearson Correlation: p = 0.0332, r = 0.4775). Error bars are SEM and colored boxes represent the full range of values, statistically significant correlations were determined by Pearson Correlation, p < 0.05, and linear regressions are displayed with 95% confidence interval bands. n = 4 to 7 mice per diet/exercise group
Discussion
Our study indicates that exercise can mitigate HFHS diet-induced subendothelial matrix stiffening, but it does not significantly affect arterial stiffness as measured on the macro-scale by PWV. These data suggest that mechanical changes induced by exercise in the intima layer are not occurring to the same extent as in the entirety of the vessel wall. Previously, we found that PWV increases and decreases following HFHS and return to a ND after HFHS, respectively, which paralleled subendothelial matrix stiffening in mice [6]. This same trend exists in aged mice that have exercised, where exercise-mediated de-stiffening occurs on both the macro- and micro-scale and PWV and subendothelial matrix stiffness are linearly correlated [14]. While PWV remains a clinical standard of arterial stiffness measurement [2], our study underscores that changes in subendothelial matrix stiffness may not be reflected in measurement of arterial stiffness by PWV. Both micro- and macro-scale measurements are important to fully characterize the biomechanical properties of blood vessels. We further emphasize the importance in performing an exercise regimen following HFHS diet consumption, as exercise can lead to de-stiffening in the subendothelial matrix layer, even when current clinical metrics (such as PWV) may not reflect these changes.
While we did not measure the direct effects of diet and exercise on endothelial cells in this work, previous studies have demonstrated benefits of exercise following a Western diet [7, 8]. Interestingly, these changes in endothelial cell phenotype have also been observed in the absence of significant changes in macro-scale arterial stiffness [7, 8]. In these studies, following exercise, Western diet-fed young mice show improvement in endothelial cell contractility and stiffness [8], and improvement in endothelium-dependent dilation [7]. One possible explanation for the changes to endothelial cell phenotype indicated by these studies [7, 8] is through altered subendothelial matrix stiffness. In our study, the subendothelial matrix stiffens on average ~ 11 kPa due to the HFHS diet, and is mitigated by 7.7 kPa with return to the ND and by 6.9 kPa with the exercise regimen. Such magnitude range changes in substrate stiffness are known to alter endothelial cell-cell junction width, cellular contraction, monolayer gaps and neutrophil transmigration in previous studies [9–11, 21]. Therefore, our data suggest that exercise, even in the absence of changes in macro-scale stiffness, may improve vascular health through altered endothelial cell monolayer function. Our data further emphasize the importance of understanding the specific contribution of subendothelial matrix mechanics to vascular health.
Our micro-scale mechanical data also indicate that the elastic modulus of the subendothelial matrix in the mouse thoracic aorta is loading rate-dependent. While the whole artery is known to demonstrate viscoelastic properties [22], the nature of the viscous and elastic contributions of the subendothelial matrix layer itself remain unknown. The intima layer is a complex tissue that contains type I collagen and type IV basement membrane collagen [23] and is bordered by the internal elastic lamina [24]. In this study, while we did not assess the distinct viscous and elastic components of the subendothelial matrix material directly, our findings suggest that the observed loading-rate dependence may be caused by the complex composition of the subendothelial matrix.
Based on our results, HFHS diet-induced stiffening is best mitigated through diet reversal, as this intervention had a profound effect on both micro- and macro-scale stiffness in accordance with our previous findings [6]. The effects of exercise on arterial stiffness after HFHS consumption is complex. Clinical studies have shown that PWV decreases following a hypocaloric diet, but does not change after low-intensity resistance exercise training alone [25], and that PWV may not show a significant change after exercise and diet lifestyle modifications [26]. These studies, along with our data, suggest that a change in diet is more effective for decreasing PWV than exercise and has the added impact of reducing subendothelial matrix stiffening.
The relationship between blood pressure (BP) and arterial stiffness is complex, and groups have emphasized the importance of its measurement in exercise studies with bulk arterial analysis [16, 20]. Due to the nature of PWV as a bulk mechanical measurement in live animals, it may be affected by structural arterial remodeling and/or vasoactive factors [19]. In fact, PWV, BP, and heart function are all intertwined [27, 28]. Our current finding, that HFHS-induced PWV increases independently of MAP after two months on the HFHS diet, are in accordance with our previous work showing that arterial stiffening is significantly increased after two months of HFHS diet while the first significant increases in systolic hypertension were evident after six months [6]. Although different methods were used to assess blood pressure (tail cuff in the current versus radiotelemetry in the previous study [6]), and the intertwined relationship between PWV and blood pressure [17], at the conclusion of the study, we found a linear trend between PWV and MAP, indicating the importance of assessing BP together with arterial stiffness to provide a thorough analysis of vascular function.
In this study, we find that exercise mitigates micro-scale subendothelial matrix stiffness, but does not impact the mechanics of the whole vessel wall to the same extent. While a majority of research efforts focus on elucidating cellular and molecular mechanisms for macro-scale vessel stiffening, the underlying causes of altered subendothelial matrix stiffness remain unanswered. To further investigate the possible causes of subendothelial matrix stiffening with HFHS diet, extracellular matrix components such as collagen and elastin should be analyzed, particularly with respect to their content in the subendothelial matrix layer, as alterations in these components are associated with arterial stiffening [8, 29]. Recently, profibrotic cytokines which are implicated in collagen synthesis, transforming growth factor-β (TGF-β) and connective tissue growth factor (CTGF), were demonstrated to be downregulated by exercise treatment in HFHS diet-fed mice, specifically in the endothelial layer [8]. Another possible cause for subendothelial matrix stiffening is through the action of advanced glycation end products (AGEs), which have previously been shown to affect the extracellular matrix structure and decrease following exercise in aged [30] or obese and diabetic [31] rats. As AGEs work through a myriad of mechanisms, such as through crosslinking collagen and elastin fibers [32], we propose that future studies should examine the roles that AGEs may have in altering subendothelial matrix stiffness. We have also previously demonstrated that inflammatory cytokines tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, and macrophage inflammatory protein (MIP)-1α mRNA are upregulated after consumption of a HFHS diet and are significantly reduced following a return to ND consumption [6]. Therefore, future studies are warranted to determine the effect of an exercise treatment on these inflammatory mediators in relation to subendothelial matrix stiffness.
Following HFHS diet consumption, the exercise period used in this study was successful at micro-scale arterial de-stiffening, but did not significantly cause decreased macro-scale arterial stiffness. PWV may further decrease with a longer swim period, or a different type of exercise, although the literature demonstrates different outcomes from different exercise regimens [7, 8]. In apolipoprotein-E deficient mice on a high-fat diet, swimming for 8 or 16 weeks has proven to be effective in reducing fatty streaks or fibrofatty plaques, respectively [33]. However, in our study, swimming for 8 weeks was sufficient to reduce subendothelial matrix stiffness, but not PWV in mice remaining on a HFHS diet. Together, these data suggest that even in the absence of changes to the macro-scale stiffness of the vessel, exercise may help reduce atherosclerosis through changes to intimal stiffness.
Conclusions
We demonstrate that following a high-fat, high-sugar diet, both exercise and return to a healthy diet decrease subendothelial matrix stiffness, while only diet reversal decreases whole vessel stiffening. The mechanical properties of the intima are influenced by factors that may not be detected by pulse wave velocity; continued research with focus on the role of subendothelial matrix stiffness is needed since intimal mechanics directly influence endothelial function and atherogenesis.
Acknowledgments
This work was supported by a grant from the National Science Foundation (award number 1435755) to C.A.R. Support was also provided by the Graduate Research Fellowship Program to J.C.K. (2013165170) under Cornell University National Science Foundation Grant DGE-1144153. This work made use of the Cornell Center for Materials Research Shared Facilities which are supported through the National Science Foundation MRSEC program (DMR-1120296) for atomic force microscopy data collection. Imaging data was acquired in the Cornell Biotechnology Resource Center-Imaging Facility using the shared, National Institutes of Health-funded (S10OD016191) VisualSonics Vevo-2100 ultrasound.
Footnotes
Conflict of Interest
Authors J.C.K., J.A., F.S. and C.A.R-K. declare that they have no conflict of interest.
No human studies were carried out by the authors for this article.
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
References
- 1.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BioMed Central Med. 2013 doi: 10.1016/S1471-4906(00)01848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell GF, Shih-jen H, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender SBB, Castorena-Gonzalez JAA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Hear Circ Physiol. 2015 doi: 10.1152/ajpheart.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM, Pasternak R, Greenland P, Smith S, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations. Circulation. 1999 doi: 10.1161/01.CIR.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 5.Kohn JC, Lampi MC, Reinhart-King CA. Age-related vascular stiffening: causes and consequences. Frontiers in Genetics. 2015 doi: 10.3389/fgene.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padilla J, Ramirez-perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA, Sowers JR. Regular exercise reduces endothelial cortical stiffness in Western diet – fed female mice. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011 doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VWM, Carman CV, Brain JD, Fredberg JJ, Butler JP, van Nieuw Amerongen GP. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol. 2011 doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroka KM, Aranda-Espinoza H. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood. 2011 doi: 10.1182/blood-2010-11-321125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lusis A. Atherosclerosis. Nature. 2000 doi: 10.1038/35025203. [DOI] [Google Scholar]
- 13.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 Update. Circulation. 2016 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 14.Kohn JC, Chen A, Cheng S, Kowal DR, King MR, Reinhart-King CA. Mechanical heterogeneities in the subendothelial matrix develop with age and decrease with exercise. J Biomech. 2016 doi: 10.1016/j.jbiomech.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peloquin J, Huynh J, Williams RM, Reinhart-King CA. Indentation measurements of the subendothelial matrix in bovine carotid arteries. J Biomech. 2011 doi: 10.1016/j.jbiomech.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Niederhoffer N, Kieffer P, Desplanches D, Sornay M-H, Atkinson J, Lartaud-idjouadiene I. Physical exercise, aortic blood pressure, and aortic wall elasticity and composition in rats. Hypertension. 2000 doi: 10.1161/01.HYP.35.4.919. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EJ, Park CG, Park JS, Suh SY, Choi CU, Kim JW, Kim SH, Lim HE, Rha SW, Seo HS, Oh DJ. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. J Hum Hypertens. 2007 doi: 10.1038/sj.jhh.1002120. [DOI] [PubMed] [Google Scholar]
- 19.Sacre JW, Jennings GLR, Kingwell BA. Exercise and dietary influences on arterial stiffness in cardiometabolic disease. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.113.02277. [DOI] [PubMed] [Google Scholar]
- 20.Steppan J, Sikka G, Jandu S, Barodka V, Halushka MK, Flavahan NA, Belkin AM, Nyhan D, Butlin M, Avolio A, Berkowitz DE, Santhanam L. Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc. 2014 doi: 10.1161/JAHA.113.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampi MC, Guvendiren M, Burdick JA, Reinhart-King CA. Photopatterned hydrogels to investigate endothelial cell response to matrix stiffness heterogeneity. ACS Biomater Sci Eng. 2017 doi: 10.1021/acsbiomaterials.6b00633. [DOI] [PubMed] [Google Scholar]
- 22.Learoyd BM, Taylor MG. Alterations with age in the viscoelastic properties of human arterial walls. Circ Res. 1966 doi: 10.1161/01.RES.18.3.278. [DOI] [PubMed] [Google Scholar]
- 23.Murata K, Motayama T, Kotake C. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis. 1986 doi: 10.1016/0021-9150(86)90172-3. [DOI] [PubMed] [Google Scholar]
- 24.Gerrity R, Cliff W. The aortic tunica intima in young and aging rats. Experimental and Molecular Pathology. 1972 doi: 10.1016/0014-4800(72)90012-3. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa A, Vicil F, Sanchez-Gonzalez MA, Wong A, Ormsbee MJ, Hooshmand S, Daggy B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am J Hypertens. 2013 doi: 10.1093/ajh/hps050. [DOI] [PubMed] [Google Scholar]
- 26.Miyatake N, Sakano N, Saito T, Numata T. Changes in exercise habits and pulse wave velocity with lifestyle modification in Japanese. Open J Epidemiol. 2012 doi: 10.4236/ojepi.2012.22008. [DOI] [Google Scholar]
- 27.Dart AM, Kingwell BA. Pulse pressure- a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001 doi: 10.1016/S0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 28.Regnault V, Lagrange J, Pizard A, Safar ME, Fay R, Pitt B, Challande P, Rossignol P, Zannad F, Lacolley P. Opposite predictive value of pulse pressure and aortic pulse wave velocity on heart failure with reduced left ventricular ejection fraction. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.02046. [DOI] [PubMed] [Google Scholar]
- 29.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals D. Rodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Q, Wang B, Zhang X-F, Ma Y-P, Liu J-D, Wang X-Z. Contribution of receptor for advanced glycation end products to vasculature-protecting effects of exercise training in aged rats. Eur J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Boor P, Celec P, Behuliak M, Grančič P, Kebis A, Kukan M, Pronayová N, Liptaj T, Ostendorf T, Šebeková K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009 doi: 10.1016/j.metabol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 33.Okabe T, Shimada K, Hattori M, Murayama T, Yokode M, Kita T, Kishimoto C. Swimming reduces the severity of atherosclerosis in apolipoprotein E deficient mice by antioxidant effects. Cardiovasc Res. 2007 doi: 10.1016/j.cardiores.2007.02.019. [DOI] [PubMed] [Google Scholar]