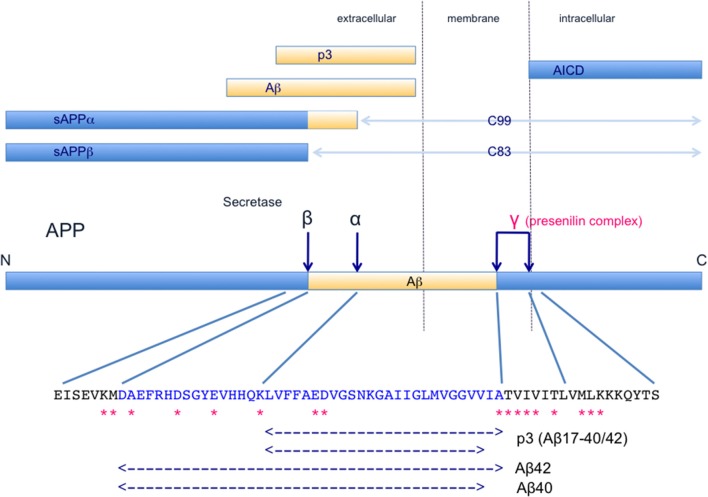
Figure 1.
Schematic illustration of the structure and metabolism of APP and its derivatives. Dark blue arrows indicate cleavage sites. α-Secretase (TACE/ADAM) (Buxbaum et al., 1998; Lammich et al., 1999) cleaves the α-site and β-secretase (BACE1, Vassar et al., 1999) cleaves the β-site, affording N-terminal fragments, sAPPα and sAPP β, and C-terminal fragments, C83 and C99, respectively. C83 and C99 are further cleaved at the γ-sites by γ-secretase complex, which includes presenilin-1, nicastrin, Aph-1 and Pen2 (Capell et al., 1998; De Strooper et al., 1998; Yu et al., 2000; Francis et al., 2002; Goutte et al., 2002; Takasugi et al., 2003). AICD and p3/Aβ are produced and released from the membrane. In the normal physiological state, α-secretase cleaves 90% or more of APP and the remaining APP is cleaved by β-secretase. Therefore, the major products in this APP metabolic pathway are sAPPα, C83, p3, and AICD, and Aβ is a minor product. AICD is rapidly degraded (Cupers et al., 2001; Kopan and Ilagan, 2004; Kametani and Haga, 2015). Thus, mutations found in familial AD, especially presenilin mutations, may affect the formation and processing of a variety of products. A part of APP sequence including Aβ is shown. Asterisks indicate APP mutations that have been identified in familial AD. These pathogenetic mutations of APP cluster near the α-secretase, β-secretase and γ-secretase cleavage sites. These mutations cause accumulation of APP C-terminal fragments (Tesco et al., 2005; Wiley et al., 2005; Xu et al., 2016a), and such accumulation has been found even in sporadic AD brains (Pera et al., 2013). Furthermore, mutations in presenilin, a constitutive protein of the γ-secretase complex, reduce γ-secretase activity (Chen et al., 2002; Walker et al., 2005; Bentahir et al., 2006; Shen and Kelleher, 2007; Xia et al., 2015). Decrease in the catalytic capacity of γ-secretase, which would lead to an increase of APP C-terminal fragments, facilitates the pathogenesis in sporadic and familial AD (Svedruzic et al., 2015).