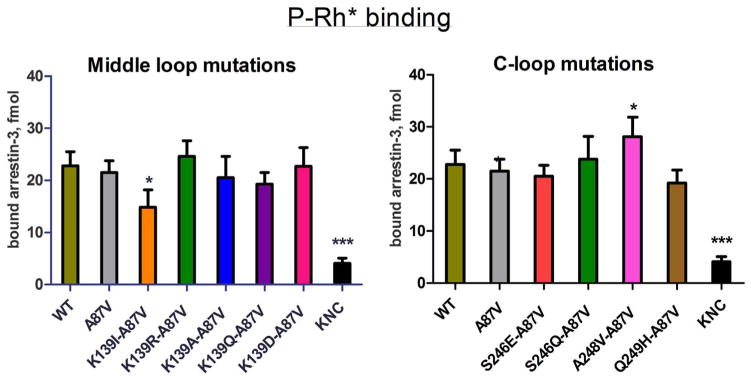
Fig. 4.
Direct binding of arrestin-3 mutants to light-activated phosphorylated rhodopsin. WT and mutant forms of arrestin-3 produced in cell-free translation in the presence of radiolabeled leucine (2 nM) were incubated with 0.3 μg of light-activated phosphorylated rhodopsin (P-Rh*) for 5 min at 37 °C. Free and rhodopsin-bound arrestin was separated by gel filtration on 2-ml Sepharose 2B–CL columns. The amount of bound arrestin eluting with rhodopsin-containing membranes was quantified by scintillation counting. Non-specific binding (in the absence of rhodopsin) was subtracted. Means ± S.D. of three independent experiments performed in duplicate are shown. The data were analyzed by one-way ANOVA with arrestin type as the main factor, followed by Dunnett’s post-hoc test.