Figure 5. Differential binding modes of EDE versus FLE antibodies (Abs).
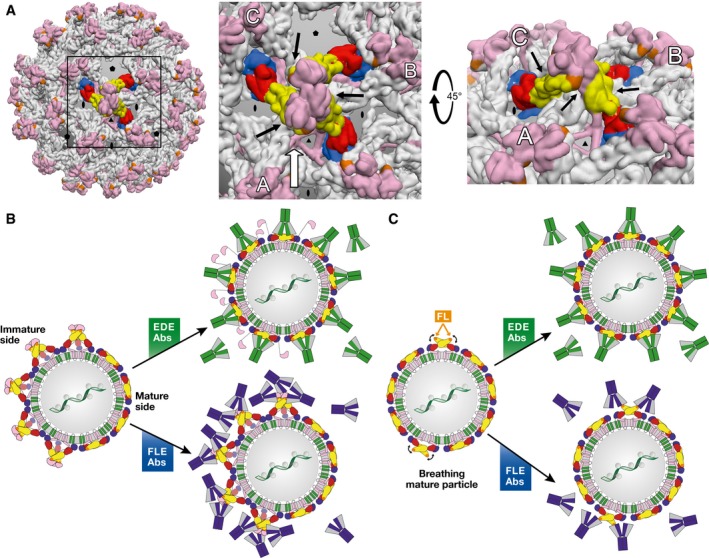
(A) Left panel: Intertwined lattice of (prM/E)3 trimers in the spiky immature particles. A central (prM/E)3 spike is represented with protein E colored by domains as in Fig 1. All other spikes have protein E in gray. prM is displayed in pink throughout. A box highlights the region zoomed in the middle panel. Middle panel: intertwined array of (prM/E)3 spikes, in which the three adjacent trimers (A, B, and C) interact underneath the central one (black arrows), underpinning it. In partially immature spikes, such stabilization is lost for the trimers at the interface between spiky and mature patches. Right panel: View along the white arrow of the middle panel, rotated by 45°. The black arrows point to the contacts between adjacent spikes. (B) Interaction of partially mature particles with EDE (green) and FLE (blue) antibodies. The spikes at the interface between mature and immature patches do not have the underpinning and stabilizing effect of three surrounding spikes shown in (A), and are therefore destabilized at these edge regions. The very high affinity of the EDE antibodies for the E dimer appears to shift the trimer‐dimer equilibrium toward dimers at the interface. Inner trimers become destabilized in turn upon EDE antibody binding, resulting in a domino effect such that the particle ends up fully coated with antibodies, as illustrated in the middle, top panel. FLE antibodies, in contrast, can readily bind to the immature patches, but also to the mature side depending on the extent of breathing (see panel C). (C) Interaction of mature particles with EDE (green) and FLE (blue) antibodies. Particle breathing (indicated by curved black arrows) leads to transient exposure of the fusion loop. EDE antibodies bind readily, regardless of the breathing behavior of the E dimers. FLE antibodies can only bind when the fusion loop is exposed through breathing, allowing particle internalization via Fcγ receptor‐mediated endocytosis before attaining sufficient antibody coating for neutralization depending on the breathing kinetics, giving rise to ADE. This situation can also explain why neutralizing antibodies, if present with ADE‐prone antibodies such as the FLEs, can override ADE by completing the antibody coating, without the need to displace the bound FLE antibody.
