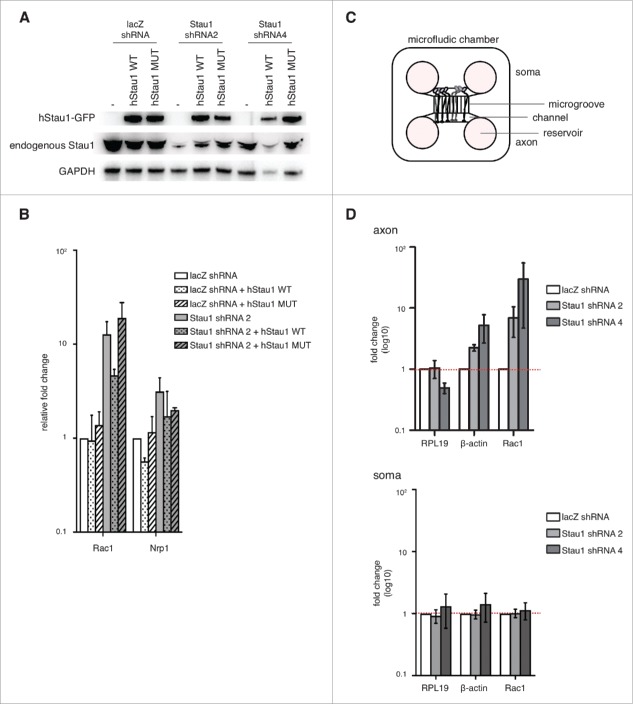
Figure 4.
Staufen1 is required for local RNA decay in the DRG neurons. A. Expression of wild-type and mutant Staufen1. E14 DRG neurons were depleted of Staufen1 using lentivirus expressing Staufen1-specific shRNA. Staufen1 depletion was rescued by expression wild-type Staufen1 (WT) or a Staufen1 mutant (MUT) that can transport mRNA but cannot mediate SMD. Lysates were immunoblotted to examine Staufen1 and GAPDH (load control) expression levels. As shown in the immunoblots, ectopic Staufen1 WT and MUT were successfully expressed in the neurons. B. An SMD-deficient Staufen1 is unable to rescue Staufen1 depletion. Staufen1 depletion causes increased expression of Rac1 and Nrp1. We rescued Staufen1 depletion with wild-type Staufen1 and a mutant that lacks SMD activity.27 Total RNA was extracted and18S rRNA, RPL19, Rac1 and Nrp1 were detected by qRT-PCR. As expected, Rac1 is upregulated when Staufen1 is depleted. When Staufen1 WT is used to rescue the knockdown, Rac1 levels are reduced towards the level in control neurons. In contrast, when Staufen1 MUT is expressed, the Rac1 amount remained similar to the Staufen1 depletion. This indicates that Staufen1 MUT cannot reverse Rac1 upregulation caused by Staufen1 depletion. This suggests that Staufen1's SMD is required for its ability to regulate Rac1 expression. C. Schematic of microfluidic chambers using for isolating axons. The schematic diagram shows the cell body (left) and axonal (right) compartments separated by 450 µm-long microgrooves. The microgrooves are small enough to allow axons to travel through, but prevent cell bodies from appearing in the axonal compartment. Approximately 20,000–30,000 neurons are plated in the cell body compartment, with axons reaching the axonal compartment by DIV2. D. Rac1 and β-actin are SMD targets in axons. Axonal transcript levels were detected by qRT-PCR after isolation of axons prepared from neurons cultured in microfluidic chambers. Three to four days after plating neurons in microfluidic chambers, lentivirus was added to the neurons to deplete Staufen1. Total RNA was extracted from the axons and the soma. qRT-PCR was performed on axonal and soma cDNA samples to probe β-actin and Rac1 (white: control, light grey: shRNA2, dark grey: shRNA4). Axonal β-actin and Rac1 show an increase in transcript levels compared to the control neurons when Staufen1 is depleted. In contrast, somatic β-actin and Rac1 transcript levels do not increase compared to the control neurons when Staufen1 is depleted. This indicates that Staufen1 is required to properly regulated β-actin and Rac1 in the DRG axons. This further suggests that SMD functions locally in the axons.