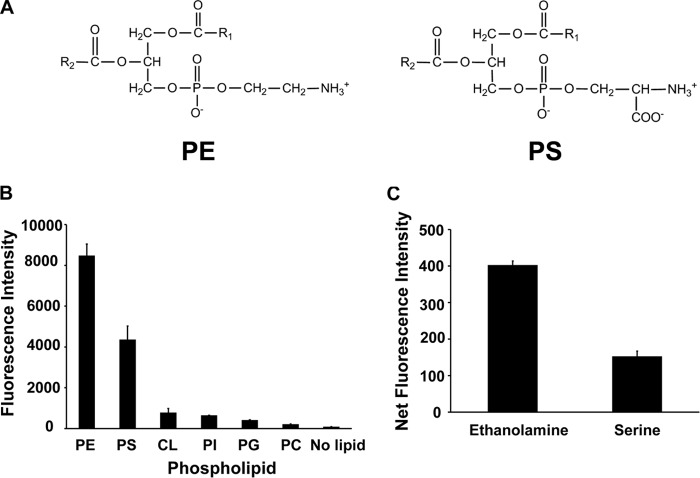
Figure 2.
DSB-3 reacts with PE and PS to produce a relatively strong fluorescent signal. A, structure of PE and PS. B, relative fluorescence intensity after reaction of PE, PS, and the non-primary amine-containing phospholipids, cardiolipin (CL), phosphatidylinositol (PI), phosphatidylglycerol (PG), and phosphatidylcholine (PC), with DSB-3. The phospholipids (0.5 mm in 0.25 mm Triton X-100) were mixed with DSB-3 (10 μm) for 1 h at 22 °C in 10 mm sodium tetraborate buffer (pH 9.5) in black-sided microtiter wells. Fluorescence was measured using a Tecan microplate reader with λex = 403 nm and λem = 508 nm. C, ethanolamine (10 mm) and serine were mixed with DSB-3 (10 μm) for 20 min at 22 °C, and fluorescence was measured using λex = 400 nm and λem = 490 nm. The data are from three independent experiments, and values are means ± S.E. (error bars).