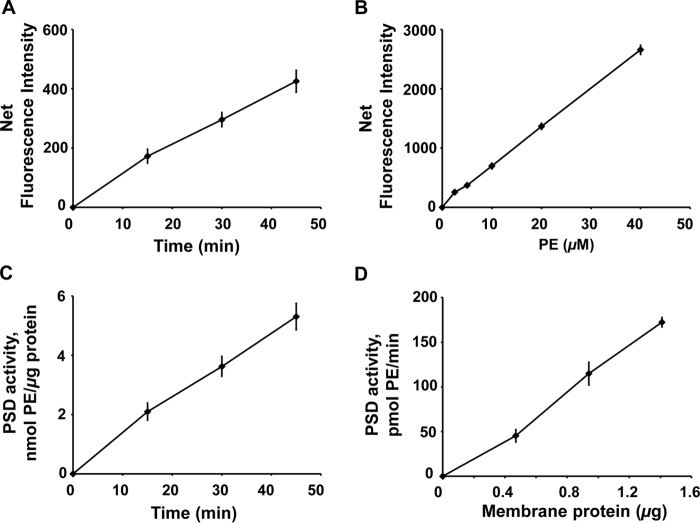
Figure 9.
Application of DSB-3–based fluorescence PSD assay to membrane fractions purified from E. coli. A, E. coli PSD enzyme assays were performed with a membrane fraction (14.1 ng of protein/μl) prepared by the methods outlined in Fig. 8A. After the PSD reaction, fluorescent adduct formation with DSB-3 was initiated as described in the legend to Fig. 8B. After 120 min, the fluorescence was quantified. A, net fluorescence intensities of PE adducts are shown after background correction. Background fluorescence was determined with heat-inactivated membranes using the approach described in the legend to Fig. 8B. B, standard curve of DSB-3 fluorescence with PE/PS mixed micelles produced in the presence of heat-inactivated E. coli membranes. Mock PSD assays of heat-inactivated cell extracts were performed with the mixed PE/PS micelles, varying the lipid ratios from 0 to 100%, with the total phospholipid content of the micelles maintained at 0.5 mm. Fluorescence detection was performed after dilution of the mixture 12.5-fold. Net fluorescence intensities of increasing PE content in the mixed PE/PS micelles are calculated after background correction with the fluorescence value of the 0 mm PE, 0.5 mm PS. C, PSD activity of the E. coli cell-free extracts was calculated by applying the fluorescence data in A and B. Net fluorescence intensities of increasing PE in the mixed PE/PS micelles are calculated after background correction with fluorescence value of the 0 mm PE. The data are from six independent experiments and are means ± S.E. D, PSD activities of the E. coli membrane fractions are shown after conducting the PSD assay with varying amounts of membrane fraction (4.7–14.1 ng of protein/μl) for 45 min. The data are from five independent experiments and are means ± S.E. (error bars).