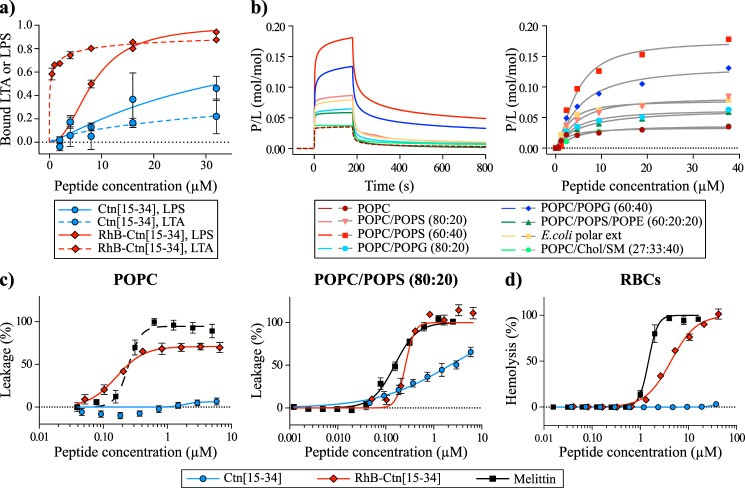
Figure 5.
Interaction of Ctn(15–34) and RhB-Ctn(15–34) with LPS, LTA, model membranes, and RBCs. a, binding of Ctn(15–34) and of RhB-Ctn(15–34) to LTA and LPS as examined using the LAL assay. The concentration of RhB-Ctn(15–34) to neutralize 50% of LTA and LPS is 0.22 and 8.26 μm, respectively. Ctn(15–34) requires >32 μm to neutralize both LTA and LPS. b, binding of Ctn(15–34) to model membranes followed by surface plasmon resonance. Peptide samples were injected over lipid bilayer deposited onto an L1 chip surface; sensorgrams (left) obtained upon injection of 38 μm peptide over a particular lipid bilayer for 180 s (association phase) and the dissociation followed for 600 s (dissociation phase). Shown is the amount of Ctn(15–34) (right) obtained at the end of association phase (t = 170 s) upon injection of various peptide concentrations. The signal of sensorgrams and dose–response curves were normalized to peptide/lipid ratios (P/L, mol/mol) by converting response units into mol of peptide and normalized for the amount of lipid deposited onto the lipid surface (1 response unit = 1 pg/mm2 of peptide or lipid). c, membrane disruption induced by Ctn(15–34) and RhB-Ctn(15–34). The percentage of membrane leakage was determined by CF dequenching. LUVs composed of POPC or POPC/POPS (80:20) with total lipid concentration of 5 μm were incubated with various concentrations of peptide. Melittin, a membrane-disrupting peptide, was included as a positive control. d, toxicity against RBCs induced by Ctn(15–34) and RhB-Ctn(15–34). A suspension of RBCs (0.25%, v/v) was incubated with various concentrations of peptides. Percentage of hemolysis was detected by absorbance of hemoglobin released into the supernatant. Melittin was included as a control peptide with hemolytic properties. Error bars, S.D.