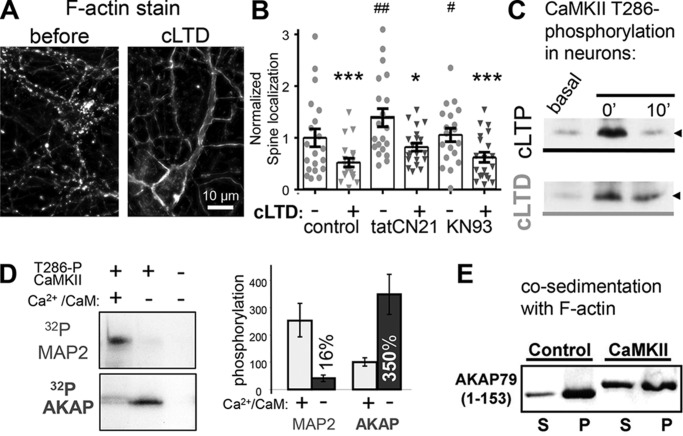
Figure 2.
F-actin re-organization during cLTD is unaltered by CaMKII inhibition. A, Texas Red phalloidin F-actin stain of DIV 12 hippocampal neurons before and 10 min after cLTD (30 μm NMDA). B, percent of F-actin synaptic localization from experiments from three individual neuron preparations, as determined by defining synaptic puncta using threshold masks as described under the “Experimental procedures.” cLTD-induced F-actin removal from spines as indicated by decreased percentage of F-actin in puncta was significant for control (before 1 ± 0.17 and after 0.5 ± 0.08, n = 19 neurons, ***, p < 0.001), tatCN21- (before 1.4 ± 0.17 and after 0.8 ± 0.07, n = 17 neurons, *, p < 0.05), and KN93 (before 1.1 ± 0.13 and after 0.6 ± 0.1, n = 19 neurons, ***, p < 0.001)-treated neurons as assessed by one-way ANOVA, Newman-Keuls post hoc analysis. Basally, tatCN21 and KN93 both stabilized F-actin in spines compared with control (##, p < 0.01; #, p < 0.05, respectively, one-way ANOVA, Newman-Keuls post hoc analysis). C, induction of the CaMKII Thr-286 autophosphorylation that generates autonomous activity after cLTP or cLTD stimuli of hippocampal cultures, assessed by Western blot analysis. The arrowhead marks 50 kDa. D, in contrast to regular substrates like MAP2, AKAP79/150 is phosphorylated more efficiently by autonomous versus Ca2+/CaM-stimulated activity. Both MAP2 and the AKAP79-targeting domain were present in the same radioactive phosphorylation assays; shown are autoradiographs after SDS-PAGE (left) and quantification of three experiments (right; mean ± S.E.). E, phosphorylation of the AKAP79-targeting domain by autonomous CaMKII reduces its interaction with F-actin in vitro, as assessed by a co-sedimentation assay. Shown is a Western blot of the supernatants (S) and pellets (P) after centrifugation at 100,000 × g that sediments F-actin together with bound proteins.