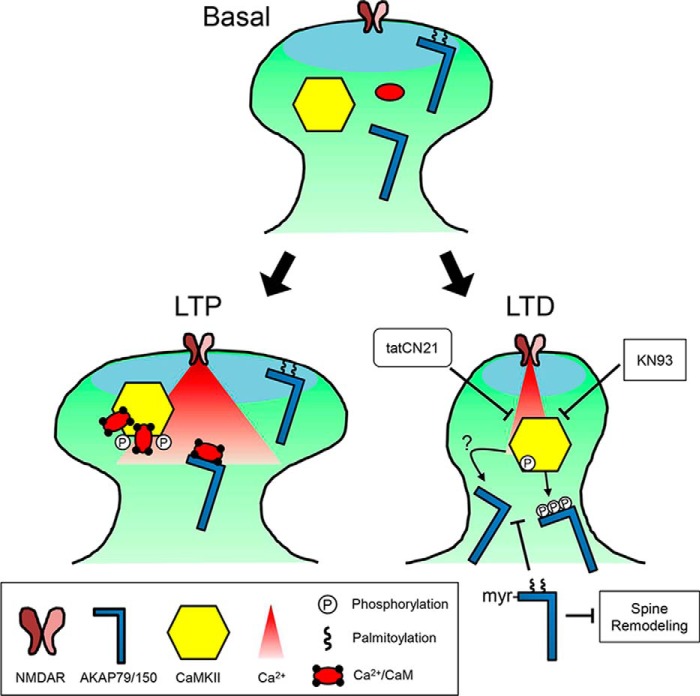
Figure 8.
Model for the LTD-specific removal of AKAP79/150 by CaMKII signaling. Under basal conditions, a pool of AKAP79/150 is palmitoylated and present in dendritic spines. During brief, strong Ca2+ influx that induces LTP, any depalmitoylated AKAP is protected from phosphorylation by Ca2+/CaM-stimulated CaMKII activity due to Ca2+/CaM binding to the targeting domain. In contrast, following weak and extended Ca2+ influx, such as elicited by NMDA-LTD induction, autonomous CaMKII activity promotes AKAP79/150 depalmitoylation, removal from spines, and spine shrinkage. These effects are accomplished in part by phosphorylation of AKAP79/150's basic membrane target domains (which is no longer protected by Ca2+/CaM) but likely require additional pathways that block DHHC PAT and/or activate PPT enzymatic activities. Inhibitors of CaMKII block NMDA-induced AKAP79/150 depalmitoylation, and ectopic lipidation of AKAP79/150 blocks its NMDA-induced removal from spines and spine structural remodeling.