Figure 7.
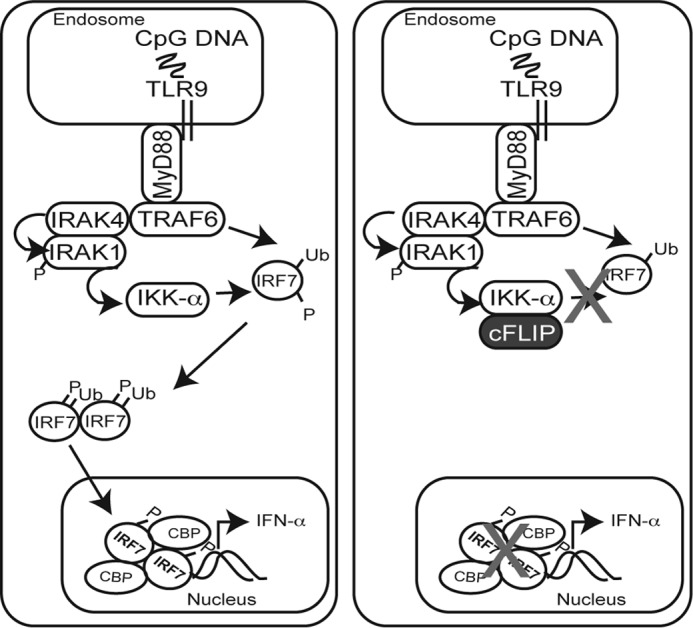
Proposed mechanism for cFLIP-mediated inhibition of IRF7-driven IFNα production. Activation of endosomal TLRs such as TLR7 by single-stranded RNAs and TLR9 by CpG motifs (e.g. CpG-A) leads to recruitment of the MyD88 protein. Next is the formation of a dynamic complex including at least IRAK4, IRAK1, and TRAF6. This complex triggers TRAF6-mediated Lys-63–linked ubiquitination of IRF7, followed by IRF7 phosphorylation. A current favored model proposes that IRAK4 phosphorylates IRAK1, leading to phosphorylation of IKKα. IKKα, in turn, activates IRF7. Phosphorylated IRF7 homodimerizes and translocates to the nucleus, where it drives expression of IFNα. The data shown here suggest that cFLIP binds to IKKα in a manner that prevents IKKα-mediated IRF7 phosphorylation and subsequent downstream IRF7 action.
