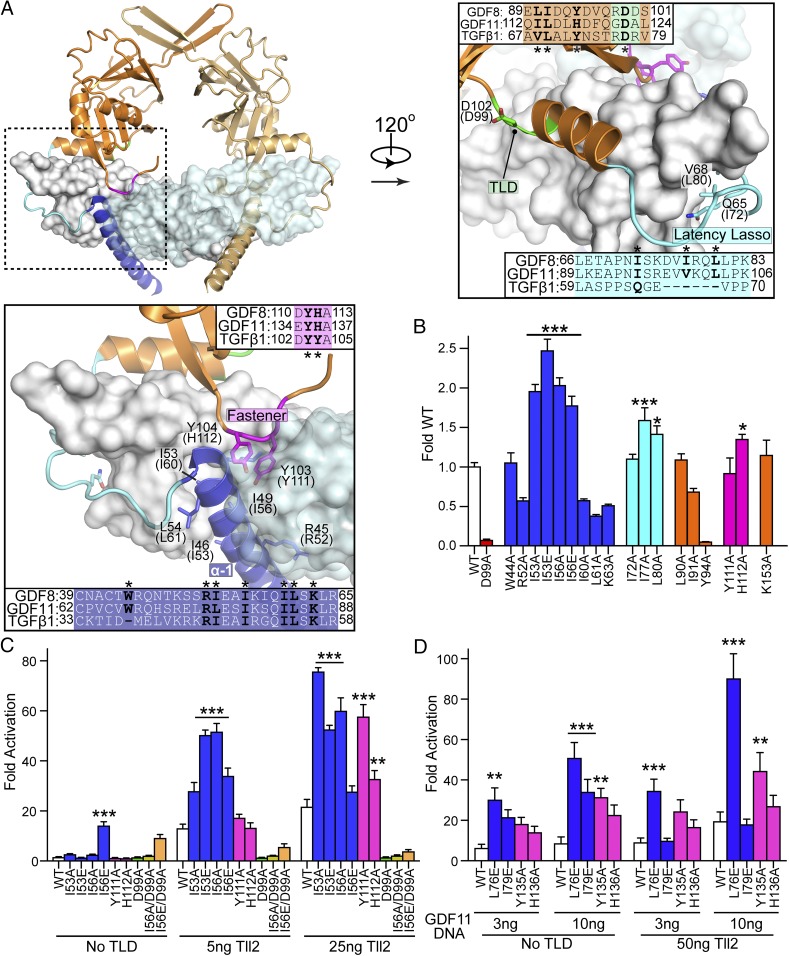
Fig. 3.
Mutations within the GDF8 prodomain and activation by Tolloid. (A) Latent TGF-β1 structure highlighting inhibitory elements of the prodomain: alpha-1 helix (blue), latency lasso (cyan), and the fastener (magenta). Sequence alignment between TGF-β1, GDF8, and GDF11 are shown with residues investigated marked with an asterisk. Labels correspond to TGF-β1 with human GDF8 in parentheses. (B) Transfection assay to determine GDF8 mutant activity. HEK293 (CAGA)12 cells were cotransfected with 25 ng of each GDF8 mutant, 50 ng of furin (human), and 25 ng of Tll2 (human) DNA. Fold activation was determined by dividing the signal from no GDF8 plasmid (25 ng of empty vector, 50 ng of furin, and 25 ng of Tll2). (C) HEK293 (CAGA)12 cells were cotransfected with 25 ng of GDF8 mutant DNA, 50 ng of furin, 25 ng of empty psF-IRES, and 0, 5, or 25 ng of Tll2. The luciferase signal was normalized to Renilla. Fold activation was calculated similar to B. (D) Transfection assay to determine GDF11 mutant activity. HEK293 (CAGA)12 were cotransfected with 3 or 10 ng of the GDF11 mutant DNA and 50 ng of furin, with or without 50 ng of Tll2. Transfection, normalization, and fold activation were calculated as they were in C. All mentioned experiments were performed at least twice where individual points were measured in triplicate. Error is shown as mean ± SEM. Bar graphs were compared using one-way (B) or two-way (C and D) ANOVA with Bonferroni correction against WT (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).